- EN - English
- CN - 中文
In situ, Cell-free Protein Expression on Microarrays and Their Use for the Detection of Immune Responses
基于原位无细胞蛋白表达的蛋白质微阵列技术及其在免疫应答检测中的应用
(*contributed equally to this work) 发布: 2019年02月05日第9卷第3期 DOI: 10.21769/BioProtoc.3152 浏览次数: 6542
评审: Chiara AmbrogioMauro Sbroggio'Enrico Patrucco
Abstract
Until recently, whole-proteome microarrays for comprehensive studies of protein interactions were mostly produced by individual cloning and cellular expression of very many open reading frames, followed by protein isolation and purification as well as array production. To overcome this cumbersome process, we have developed a method to generate microarrays representing entire proteomes by a combination of multiple spotting and on-chip, cell-free protein expression. Here, we describe the protocol for the production of bacterial protein microarrays. With slight adaptations, however, the procedure can be applied to the proteome of any organism. Expression constructs of each gene are generated by PCR on bacterial genomic DNA followed by a common secondary amplification that is adding relevant regulative elements to either end of the constructs. The unpurified PCR-products are spotted onto the microarray surface. Full-length proteins are directly expressed in situ in a cell-free manner and stay attached to the surface without further action. As an example of a typical application, we describe here the proteome-wide analysis of the immune response to a bacterial infectious agent by characterizing the binding profiles of the antibodies in patient sera.
Keywords: Whole-proteome microarrays (全蛋白质组微阵列)Background
Protein microarrays are an excellent tool for identifying disease-associated antibody reactivity patterns since they allow the simultaneous detection of antibody binding to a large number of antigens, up to entire bacterial proteomes (Hufnagel et al., 2018) and beyond (Syafrizayanti et al., 2017). Protein microarrays used to be generated with proteins that were isolated from recombinant cellular libraries. This approach is work-intensive, time-consuming and costly, however, since it requires the individual handling of very many cells. Each gene of interest is amplified by a polymerase chain reaction (PCR), followed by cloning into an expression vector. After transformation into a cellular system–frequently Escherichia coli (E. coli)–the cell clones are grown separately from others and characterized. Subsequently, the respective overexpressed protein is isolated and purified, followed by immobilization on the microarray. Our largest array of a non-mammalian gene set consisted of 14,000 proteins (Syafrizayanti et al., 2017), for example. Therefore, producing this array would have required going through the above process 14,000 times.
In order to overcome the complex procedure, means have been developed for the production of protein microarrays by cell-free protein expression directly on the microarray surface. After the initial publication of the method (Protein in situ Arrays, PISA) (He and Taussig, 2001), several adaptations have been reported (Ramachandran et al., 2004; Angenendt et al., 2006; He et al., 2008). All have in common that genes are copied into DNA expression constructs by PCR, which are directly placed onto the microarray surface. Proteins are then synthesized in situ using cell lysates containing all elements and ingredients required for transcription and translation. All steps are done in parallel and in a largely automated manner. No cells are involved throughout. In addition, the approach eliminates the need for protein purification.
Besides many minor variations, our protocol differs from the others mainly by the fact that multiple spotting is applied to produce protein microarrays (Angenendt et al., 2006; Syafrizayanti et al., 2017). First, the DNA expression constructs are placed on planar glass slides. In a second step, each position is revisited to deposit in a second spotting event a cell-free transcription and translation mixture on top of the DNA spots. Proteins are expressed directly on the microarray slide and bind immediately. The double-spotting protocol decreases substantially the volume of cell lysate needed for protein expression since the empty surface in between spots is not covered. Concomitantly, there is less background signal in between the spots eventually. More importantly, however, the reaction space is restricted to the locations of the DNA expression constructs. In consequence, expressed protein cannot float away but binds in situ only. No additional means, such as antibodies, are required to keep the proteins attached to their exact positions. Nevertheless, even extensive washing will not remove them. Previous studies have shown that most proteins are expressed in full-length, and very many fold into a functionally active conformation. Also, the process is versatile and can be adapted to fit to a variety of applications (Syafrizayanti et al., 2017).
Besides studies on microarrays with human or parasite proteins, we have used arrays that represent entire bacterial proteomes to screen clinically characterized patient (and control) sera in order to identify antibody binding patterns that are disease-specific. New marker molecules for disease diagnosis and prognosis were discovered in this way, including markers for cancer entities that are associated with bacterial infections. In a recent study, microarrays were produced that present all proteins of Chlamydia trachomatis (Ct), for example (Hufnagel et al., 2018). Through comparison of the antibody binding patterns on the microarrays, we identified antigens that are reproducibly recognized by the antibodies in Ct-seropositive samples, in addition to molecules that were patient-specific. With samples from cervical cancer patients, we found the common Ct-specific markers and additional antigens, which were shared between the cancer patients only. Large-scale validation experiments using high-throughput suspension bead array serology on hundreds of samples confirmed the significance and relevance of the newly identified markers for both general Ct infection and related cervical cancer. Next to providing meaningful marker molecules, the results strongly support the hypothesis that there is an association of Ct infection with cervical cancer.
The quick and efficient proteome-wide immunoassay can easily be adapted to other microorganisms in all areas of infection research. By reverse transcribing RNA to DNA-templates, the protocol is applicable to any transcript isolate. We also have utilized established ORF libraries, such as the Human ORFeome library (The ORFeome Collaboration, 2016), by which the process of microarray production is simplified further since only one vector-specific primer pair is required for the initial PCR. Here, we provide a detailed protocol for the production of whole-proteome bacterial microarrays and their application to screening patient sera for the immune response in infected people.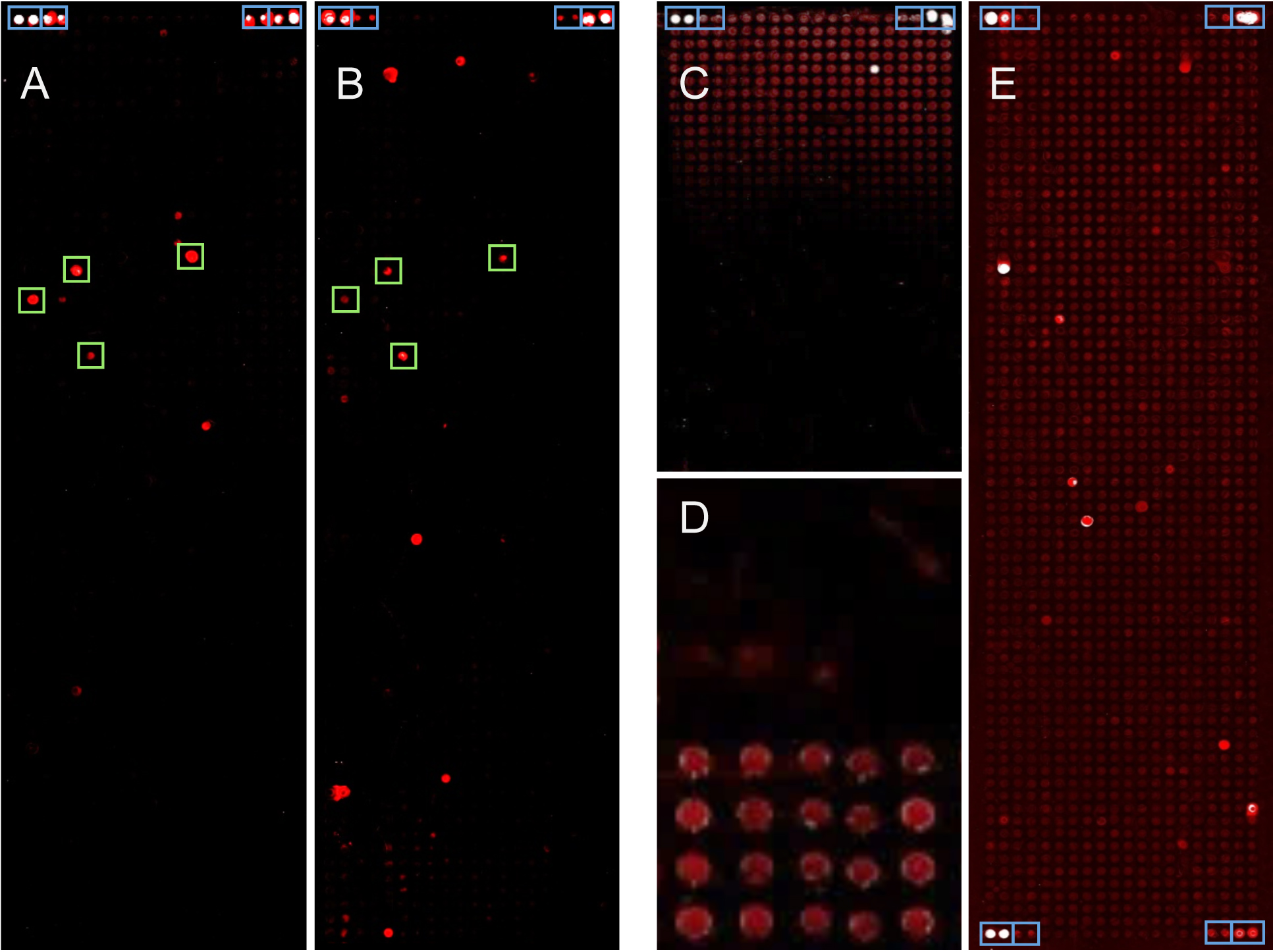
Figure 1. Images of protein microarrays made from bacterial genes. About 1,500 proteins were produced on each array. Blue boxes indicate controls. A-B. Two examples of analyses are presented that were performed using the protocol described below. Signals that were common to a group of patients are labeled by green boxes. C-E. Few examples of technical problems are presented. C. An artifact is shown, which was due to a technical fault of the spotting device, resulting in a signal gradient across the microarray. D. In a close-up, background signals on 40 microarray positions are shown. The only difference between the top and bottom half was the quality of the DNA-polymerase used for PCR. After spotting unpurified PCR-products, incubation with patient serum and subsequent labeling produced significant differences in background signal. E. A complete protein microarray is shown after incubation with patient serum and detection of antibody binding. Because of inadequate blocking, a relatively high level of background signal was produced.
Materials and Reagents
- Corning Costar Stripette serological pipettes (Corning, Sigma-Aldrich, catalog number: CLS4488-50EA)
- Epoxysilane-coated slides (Schott, catalog number: 1066643)
- Microarray hybridization cassettes (Arrayit Corporation, blue: AHCTZ, green: AHCP, gold: AHCC)
- ProPlate Slide Modules (Grace Bio-Labs, catalog number: GBL246861-2EA)
- Standard 96-well PCR plate (Steinbrenner Laborsystem, catalog number: SL-PP96-4L)
- Microplates, 384-well (Greiner Bio-One, catalog number: 781281)
- PCR seal (4titude, catalog number: 4ti-0500)
- quadriPerm chambers (SARSTEDT, catalog number: 94.6077.307)
- Escherichia coli BL21 wild-type bacteria (GE Healthcare)
- 6x DNA loading dye (Thermo Scientific, catalog number: R0611)
- Alexa Fluor 647-conjugated goat anti-human IgA, IgG, IgM (Jackson Immuno Research, catalog number: 109-605-064)
- Agarose NEEO Ultra-Quality (Carl Roth, catalog number: 2267.4)
- Betaine monohydrate (Sigma-Aldrich, catalog number: 14300)
- Bovine serum albumin (Carl Roth, catalog number: 8076.3)
- Calcium chloride (Carl Roth, catalog number: CN93.2)
- Deoxynucleotide (dNTP) solution set (New England Biolabs, catalog number: N0446S)
- Disodium phosphate (Neolab Migge GmbH, catalog number: 4770.1000)
- DL-Dithiothreitol (DTT) (Thermo Scientific, catalog number: R0861)
- Ethylenediaminetetraacetic acid (Carl Roth, catalog number: 8043.2)
- GeneRuler 1 kb DNA Ladder (Thermo Scientific, catalog number: SM0311)
- Glacial acetic acid (Sigma-Aldrich, catalog number: 695084)
- Glycine (Gerbu, catalog number: 56406)
- Nickel(II) sulfate hexahydrate (Sigma-Aldrich, catalog number: 227676)
- Nitrilotriacetic acid (NTA) (Sigma-Aldrich, catalog number: 72559)
- Nuclease-free water (Life Technologies, catalog number: AM9922)
- Monoclonal anti-V5-Cy3 antibody produced in mouse (Sigma-Aldrich, catalog number: V4014-100UG)
- Monopotassium phosphate (Carl Roth, catalog number: 3904.1)
- Penta-His Alexa Fluor 647 Conjugate (QIAGEN, catalog number: 35370)
- peqGreen (VWR, peqLab brand, catalog number: 732-3196)
- Potassium chloride (Carl Roth, catalog number: 6781.1)
- Q5 High-Fidelity DNA polymerase, 100 Units, and buffers (New England Biolabs, catalog number: M0491S)
- S30 T7 High-Yield Protein Expression Kit (Promega, catalog number: L1110)
- SuperBlock (PBS) blocking buffer (Thermo Scientific, catalog number: 37515)
- Taq DNA Polymerase (1,000 U) and buffers (QIAGEN, catalog number: 201205)
- Tris base (Sigma-Aldrich, catalog number: SLBP5070V)
- Sodium carbonate (Na2CO3) (Carl Roth, catalog number: A135.1)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888-1KG-M)
- Sodium hydrogen carbonate (NaHCO3) (Carl Roth, catalog number: 6885.1)
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 1310-73-2)
- Tryptone (Sigma-Aldrich, catalog number: T7293-250G)
- Tween-20 (Sigma-Aldrich, catalog number: P1379)
- Yeast extract (Sigma-Aldrich, catalog number: Y1625)
- Complete Protease inhibitor (Roche, catalog number: 04693132001)
- KH2PO4 (Merck, catalog number: 1048711000)
- MgCl2 (Carl Roth, catalog number: KK36.1)
- Acetic acid (Sigma-Aldrich, catalog number: A6283)
- Glycerol (Sigma-Aldrich, catalog number: G5516)
- BSA-blocking buffer (see Recipes)
- NTA-solution (see Recipes)
- Nickel-solution (see Recipes)
- LB-medium (see Recipes)
- Opti-Solution (see Recipes)
- 10x PBS (see Recipes)
- PBST (see Recipes)
- TAE (50x) (see Recipes)
Equipment
- Pipettes (Gilson, PIPETMAN Classic, catalog numbers: F144566, F144057M, F144055M)
- Slide staining and storage systems (Sigma-Aldrich, catalog number: BAF441800000)
- Biofuge, Pico (Hereaus Instruments, DJB Labcare 75003235)
- Magnetic stirrer (IKA, IKAMAG REO)
- Centrifuge 5810R (Eppendorf, model: 5810R, catalog number: 5811000010)
- Gel documentation system (Azure C200 Biosystems, catalog number: 170-8195)
- Homogenizator EmulsiFlex-C5 (AVESTIN, catalog number: 0506394)
- Laminar hood (Hereaus Instruments, model: LaminAir HB2436)
- Microcomputer electrophoresis power supply (Consort, model: E835)
- Mixmate Microplate Mixer (Eppendorf, model: 5353 000.014)
- NanoDrop spectrophotometer ND-1000 (Thermo Scientific, model: 7934)
- Nanoplotter 2.0 (GeSIM, model: 060-308)
- Orbital Shaker Unimay 1010 (Heidolph, model: 543-12310-00)
- Power Scanner (Tecan, model: 30038387)
- LifeEco Thermal Cycler (BioER, model: 685115)
- Ventilated oven (neoLab, catalog number: 7-9155)
- 4 °C refrigerator (neoLab, catalog number: 10064)
- -20 °C freezer (Bosch, model GIV21AF30)
Software
- Fuzznuc (EMBOSS, http://emboss.sourceforge.net/apps/cvs/emboss/apps/fuzznuc.html)
- Software NPC16 V2.15 (GeSiM, Radeberg, Germany)
- PowerScanner V1.2 (Tecan Trading AG, Switzerland)
- GenePix Pro 6.0 (Molecular Devices, Sunnyvale, USA)
- R Studio (R version 3.3.2, https://www.rstudio.com/)
- MELTTEMP (http://www.biology.wustl.edu/gcg/melttemp.html)
- Excel (Microsoft Cooperation, Redmond, USA)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hufnagel, K., Reininger, D., Ng, S. W., Gassert, N., Rohland, J. K., Shahryarhesami, S., Bauer, A. S., Waterboer, T. and Hoheisel, J. D. (2019). In situ, Cell-free Protein Expression on Microarrays and Their Use for the Detection of Immune Responses. Bio-protocol 9(3): e3152. DOI: 10.21769/BioProtoc.3152.
分类
癌症生物学 > 肿瘤免疫学 > 免疫学试验 > 蛋白质分析
微生物学 > 微生物-宿主相互作用 > 细菌
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














