- EN - English
- CN - 中文
Isolation of Thylakoid Membranes from the Cyanobacterium Synechocystis sp. PCC 6803 and Analysis of Their Photosynthetic Pigment-protein Complexes by Clear Native-PAGE
从蓝藻集胞藻sp. PCC 6803中分离类囊体膜并利用CN-PAGE非变性聚丙烯酰胺凝胶电泳分析光合色素蛋白复合物
发布: 2019年01月05日第9卷第1期 DOI: 10.21769/BioProtoc.3126 浏览次数: 8549
评审: Dennis NürnbergYuHitchcock

相关实验方案
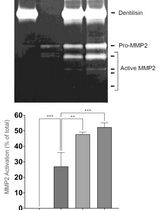
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2064 阅读
Abstract
Cyanobacteria represent a frequently used model organism for the study of oxygenic photosynthesis. They belong to prokaryotic microorganisms but their photosynthetic apparatus is quite similar to that found in algal and plant chloroplasts. The key players in light reactions of photosynthesis are Photosystem I and Photosystem II complexes (PSI and PSII, resp.), large membrane complexes of proteins, pigments and other cofactors embedded in specialized photosynthetic membranes named thylakoids. For the study of these complexes a mild method for the isolation of the thylakoids, their subsequent solubilization and analysis is essential. The presented protocol describes such a method which utilizes breaking the cyanobacterial cells using glass beads in an optimized buffer. This is followed by their solubilization using dodecyl-maltoside and analysis using optimized clear-native gel electrophoresis which preserves the native oligomerization state of both complexes and allows the estimation of their content.
Keywords: Cyanobacteria (蓝藻)Background
Thylakoids are specialized membranes of algal and plant chloroplasts and cyanobacteria. The thylakoid lipid bilayer shares characteristic features with prokaryotic bacterial membranes but it is enriched in glycolipids, namely galactolipids, while the level of acidic lipids is relatively low and does not exceed 20% of the overall lipid content. Thylakoids contain a large number of proteins and their complexes and over 40% of them are involved in light-dependent reactions of photosynthesis. The next largest functional group includes proteins involved in protein targeting, processing and folding, oxidative stress response and translation. The most integral membrane proteins of thylakoids form large protein complexes which play an important role in the harvesting of light and its photochemical conversion. The key complexes performing this conversion are PSI and PSII. They both perform the light-driven primary charge separation resulting in the thylakoid-associated flow of electrons reducing NADP+ and generating a pH gradient used for the synthesis of ATP. Both complexes are subject to intensive research focused mainly on the elucidation of their function and underlying structural features as well as the mechanism of their assembly. For this research the isolation of native membranes and their detailed analysis as concerns the content, assembly and oligomerization state of both photosystems is essential. The existing and frequently used protocols for the isolation of cyanobacterial thylakoids either do not sufficiently preserve the native structure of fragile membrane complexes like Photosystem II dimers (e.g., Komenda and Barber, 1995), or the obtained preparations contain high concentrations of compounds (for instance 1 M glycine betaine) which are not compatible with the clear native gel electrophoresis (e.g., Gombos et al., 1994). The described protocol for thylakoid preparation is optimized from both points of view. The analysis of cyanobacterial membranes by clear native PAGE (CN PAGE) using a protocol modified from Wittig et al. (2007) gives direct information about the quantity and oligomeric (or assembly) state of multisubunit pigmented photosystem complexes with unprecedented speed and resolution. The isolation, solubilization, and analysis of membranes does not disrupt weak non-covalent interactions between pigments and proteins and therefore the gel with separated individual complexes can be directly scanned. Unlike denaturing SDS gels, no fixation, staining and destaining, or, blotting and immunodetection is needed for distinguishing between PSII and PSI (see Figure 2). Green PSI complexes do not show red fluorescence at room temperature and are present as dominant green bands in the gel while PSII complexes fluoresce and their green bands are hard to distinguish on the simple transparency scan. Thus, PSI quantification is done using the simple transparency scan of green non-fluorescent bands while PSII quantification is done using the red fluorescence scan of fluorescent bands.
Materials and Reagents
- Pipette tips
- 2 ml screw cap polypropylene micro vials (Biospec Products, catalog number: 3205)
- Hamilton® microsyringe series 700, 0.25 ml (Sigma, catalog number: 24538-U)
- Hamilton needle, 51 mm (Sigma, catalog number: 21746)
- Binder Clip (Deli 8584, Luma trading)
- Magnetic stirring bars with diameter 5 mm and length 20 mm
- Transparent plastic film
- 1.5 ml spectroscopic cuvettes (Fisherbrand PS, catalog number: 7755.0302)
- The glucose tolerant strain of the cyanobacterium Synechocystis sp. PCC6803 (GT-P, Tichý et al., 2016)
- Acrylamide for molecular biology (AA) (AppliChem Panreac, catalog number: A3812,1000)
- Bisacrylamide for molecular biology (BIS) (AppliChem Panreac, catalog number: A3636,0250)
- 6-aminohexanoic acid (ACA) (AppliChem Panreac, catalog number: A2266,500)
- Bis-Tris (AppliChem Panreac, catalog number: A1025.1000)
- MES (AppliChem Panreac, catalog number: A0689.0250)
- Tricine (AppliChem Panreac, catalog number: A1085,1000)
- N,N,N',N'-tetramethylethylenediamine (TEMED) (Sigma, catalog number: T928-25ml)
- Ammonium persulfate (APS) (Sigma, catalog number: T3678-25g)
- Dithiothreitol (DTT) (AppliChem Panreac, catalog number: A1101,0025)
- SDS (Sigma, catalog number: 7172-100g)
- Glass beads with a diameter of 150-212 μm (Sigma, catalog number: G1145)
- n-Dodecyl-β-D-maltoside (DM) (AppliChem Panreac, catalog number: A0819,0005)
- Sodium deoxycholate (Sigma, catalog number: 30970-25g)
- Gel Filtration Markers Kit for Protein Molecular Weights (Sigma, catalog number: MWGF1000-1KT)
- CaCl2 (analytical grade)
- MgCl2 (analytical grade)
- Methanol (analytical grade)
- Sodium hydroxide (analytical grade)
- Hydrochloric acid (analytical grade)
- Glycerol (HPLC grade)
- Milli-Q de-ionized water (Merck Millipore, Ultrapure [Type 1] water)
- Buffer B (see Recipes)
- Gel buffer 6x (pH 7.6) (see Recipes)
- Acrylamide solution (AB) (see Recipes)
- Upper (Cathode) buffer 10x (see Recipes)
- Upper (Cathode) buffer 1x (see Recipes)
- Lower (Anode) buffer 10x (see Recipes)
- Lower (Anode) buffer 1x (see Recipes)
- Resolving gel and stacking gel (see Recipes)
Equipment
- Automatic pipettes (P1000, P200 and P10)
- Mini-beadbeater (Sigma, catalog number: Z249289)
- Magnetic stirrer Color Squid White (Thermo Fisher Scientific, catalog number: 6110.1005)
- GE gradient maker (Sigma, catalog number: GESG50)
- Vortex (Velp Scientifica, model: RX3)
- Centrifuge (Hettich, model: Micro22 R)
- UV-VIS spectrophotometer (Shimadzu, model: UV3000)
- Cooled microcentrifuge (Eppendorf, model: 5415R)
- PAGE apparatus (Bio-Rad, PROTEAN® II xi Cell)
- Power Supply (Bio-Rad, PowerPacTM HV High-Voltage)
- Cooled thermostat (Labio, model: CTB 06C)
- LAS 4000 camera (Fuji; for chlorophyll fluorescence scanning used LED blue light source 460 nm for excitation and R670 filter for emission)
- Scanner HP ScanJet G4050 (HP, model: G4050)
- pH meter (Sartorius, PB-11)
- FIM-150 flake ice maker (Biobase)
Software
- ImageJ bundled with Java 1.8.0_112 (https://imagej.nih.gov/ij/download.html)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Komenda, J., Krynická, V. and Zakar, T. (2019). Isolation of Thylakoid Membranes from the Cyanobacterium Synechocystis sp. PCC 6803 and Analysis of Their Photosynthetic Pigment-protein Complexes by Clear Native-PAGE. Bio-protocol 9(1): e3126. DOI: 10.21769/BioProtoc.3126.
分类
植物科学 > 植物生物化学 > 蛋白质 > 分离和纯化
微生物学 > 微生物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 电泳
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link