- EN - English
- CN - 中文
Quantitation of Regulatory Activity for the Complement Alternative Pathway Using an Adaptation of the AP50 in vitro Assay
应用改编过的AP50体外分析法定量测定补体旁路途径的调节活性
发布: 2018年12月20日第8卷第24期 DOI: 10.21769/BioProtoc.3116 浏览次数: 5779
评审: Vamseedhar RayaproluPaul JordanAnonymous reviewer(s)
Abstract
Complement pathways function to identify and remove pathogens and infected cells. There are three complement pathways: the classical, lectin and alternative pathway (AP). While all pathways are activated following pathogen stimuli, the AP is constitutively active and tightly controlled by activators (e.g., Factor B, Factor D) and negative regulators (e.g., Factor H). Complement activity can be measured by well-established methods that are often used in a diagnostic setting to determine the CH50 (50% complement hemolytic activity) or AP50, specifically to measure AP activity. The protocol here has adapted the traditional AP50 method designed to measure AP activity in human sera, to measure the positive or negative AP regulatory activity within a given test sample. The assay relies on the ability of AP components in human serum to lyse rabbit erythrocytes under in vitro conditions specific for the AP with subsequent release of hemoglobin that is quantitated by measurement of optical density. Our method has added test substances, such as cell culture media with defined changes in individual complement components and determined the ability to either promote or inhibit AP activity in vitro. Thus, this protocol reflects the overall functional ability of a sample to effect AP activity and can be used in the research laboratory to determine AP regulatory activity in a complex biological sample, or to test the ability of drugs or novel biomolecules to regulate AP activity.
Keywords: Complement (补体)Background
Complement is part of the innate immune system and acts to recognize and clear pathogens and stimulate adaptive immune responses. The three complement pathways, the classical (CP), lectin (LP) and alternative pathways (AP), constitute a series of cleavage and activation of complement proteins, generating enzymatically active protein complexes, such as convertase complexes, and bioactive split products, such as C3a. The three pathways converge on cleavage of the complement component C3 and one common downstream outcome is the generation of a membrane attack complex (MAC) and subsequent lysis of target cells (Ricklin et al., 2010). Functional activity of complement can be measured by assays such as the 50% complement hemolytic activity (CH50 assay), where a stimulus (antibody coated red blood cells), is provided in vitro to the complement components in a biological sample. The subsequent lysis of the target red blood cells (RBC) is quantitated by release of hemoglobin and measured by an increase in optical density. If target complement components such as C3 are missing from the test biological sample, either genetically or due to complement consumption resulting from activation of complement in vivo, then CH50 will be low. Similarly, the AP50, specifically measures the activity of the AP where rabbit RBC spontaneously activate the human AP resulting in RBC lysis (Figure 1A). The presence of EGTA chelates calcium to prevent CP activation and the addition of Mg2+, restores this requirement for the AP, yielding buffer conditions specific to determine AP activity (Joiner et al., 1983). CH50 and AP50 assays are routinely used in many diagnostic laboratories to quantitate complement activity in human blood (Prohaszka et al., 2016). In the traditional application of the AP50, a high AP activity is reflected by low availability of complement components due to complement ‘consumption’ and thus low lysis of rabbit RBC. The lysis of rabbit RBC is quantitated by detecting the release of free hemoglobin by an increase in optical density (Figure 1A). This traditional assay has been adapted here, to keep the starting complement components in the assay constant by use of one source of human serum and measure the ability of factors added in test substances to promote the cleavage of the complement components from human serum, under conditions specific for the AP (Figure 1B). In this case, with constant input of complement components, adding a sample containing a stimulator, such as factor D or B will result in increased RBC lysis, while adding a negative regulator, such as factor H (FH) will result in decreased RBC lysis. A previous study has applied a similar approach to measure complement convertase activity in human serum (Blom et al., 2014) and the method here has adapted this to measure the net stimulatory or inhibitory ability of cell culture supernatants for the AP (Cabezas et al., 2018).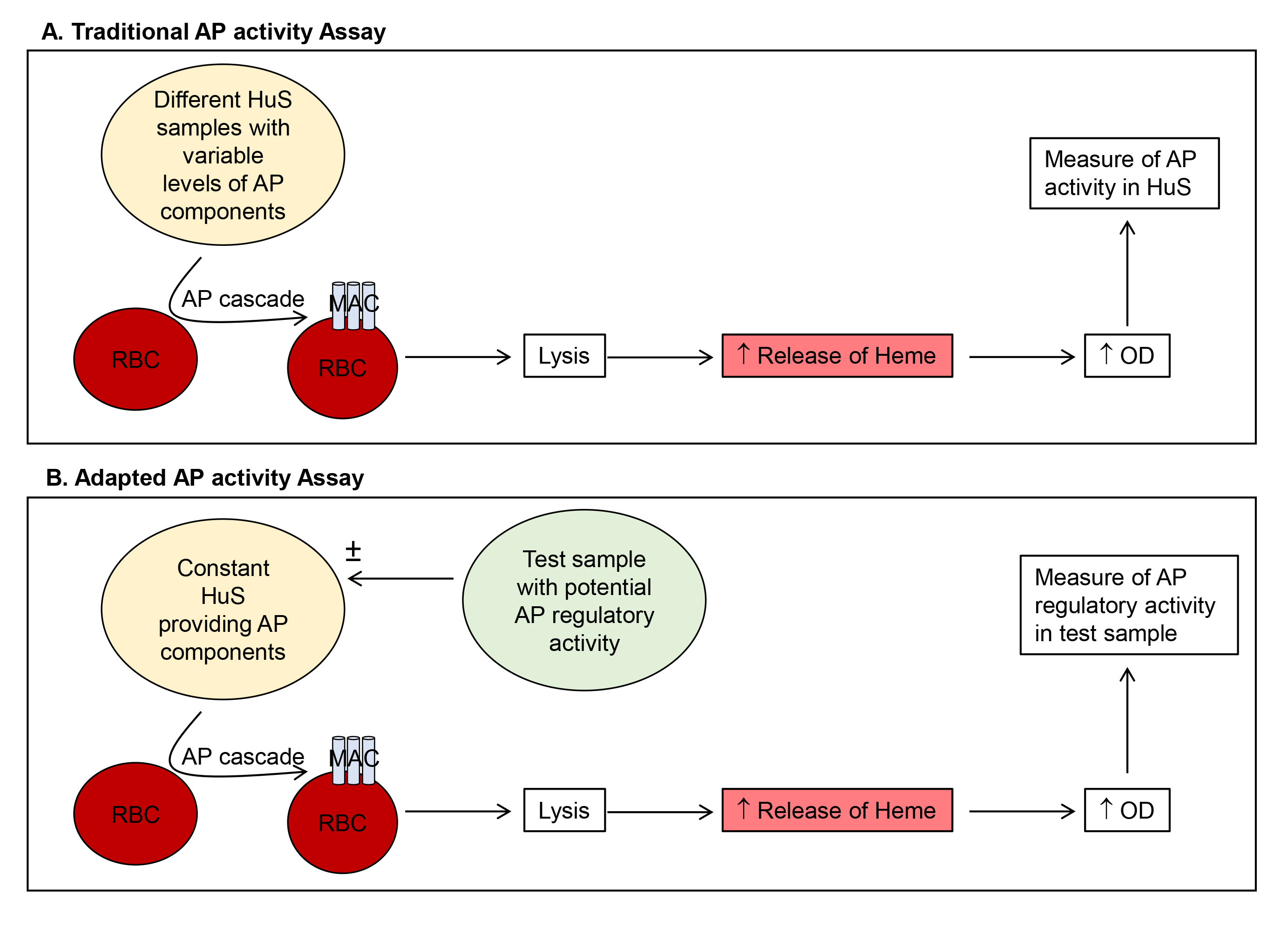
Figure 1. Comparison of traditional (A) and adapted (B) AP activity assays. Method (A) uses variable levels of HuS to quantitate AP activity in the test HuS samples. Our adapted method (B) utilizes a constant HuS sample and quantitates the AP regulatory activity of added test samples. HuS = human serum; OD = optical density.
Materials and Reagents
- Pipette tips (Axygen Scientific, catalog number: T-200-Y)
- 1.5 ml flip-topped tubes (Axygen Scientific, catalog number: MCT-150-C)
- Plastic film (Greiner multiwell plate sealer, Sigma-Aldrich, catalog number: A5596)
- Borosilicate glass beads, 5 mm (Sigma-Aldrich, catalog number: Z143944)
- 96-well flat bottomed plates (Costar, catalog number: 3596)
- Barbitone complement diluent tablets (Oxoid, catalog number: BR0016)
- NaOH pellets (UNIVAR, catalog number: A482)
- Concentrated HCl (ANALAR, catalog number: 101256J)
- Human serum (HuS), non-heat inactivated, store at -20 °C (collected from discard blood following routine clinical venesection in accordance with Southern Adelaide Local Health Network, Human Ethics Research Committee approval #343.16-HREC/16/SAC/306)
- Rabbit blood, freshly collected (under Flinders University Animal Welfare Committee approval to use scavenge material)
- Purified FH, generated in-house from human blood and stored at -20 °C [Diluted to 1 µg/µl in vehicle prior to use (Cabezas et al., 2018)]
- Vehicle = corresponding carrier for the test samples for example DMEM (Hyclone, catalog number: SH30022.01)
- Sodium chloride (NaCl) (Santa Cruz, catalog number: sc-203274A)
- Gelatin solution (2% solution) (Sigma-Aldrich, catalog number: G1393)
- EGTA (Sigma-Aldrich, catalog number: E3889)
- Veronal buffer (see Recipes)
- AP buffer (see Recipes)
- Saline (see Recipes)
- 10 N NaOH (see Recipes)
Equipment
- Pipettes (Thermo Fisher Scientific, model: Finnpipette F1)
- 50-100 ml conical flask/Erlenmeyer flask (Thermo Fisher Scientific, catalog number: KIM 265520-1)
- Oven (Labnet mini incubator)
- Hemocytometer (Counting chamber) (Hawksley, catalog number: AC1000)
- Plate centrifuge (Heraeus Sepatech Omnifuge 2.0 RS)
- Microplate reader (multimode detector) (Beckman Coulter, model: DTX880)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Falcon, S. C., Gordon, D. L. and Carr, J. M. (2018). Quantitation of Regulatory Activity for the Complement Alternative Pathway Using an Adaptation of the AP50 in vitro Assay. Bio-protocol 8(24): e3116. DOI: 10.21769/BioProtoc.3116.
分类
免疫学 > 补体分析 > 病毒
微生物学 > 微生物-宿主相互作用 > 病毒
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link