- EN - English
- CN - 中文
Generation of BMEC Lines and in vitro BMEC-HSPC Co-culture Assays
建立脑微血管内皮细胞系和体外 BMEC-HSPC 共培养检测
发布: 2018年11月05日第8卷第21期 DOI: 10.21769/BioProtoc.3079 浏览次数: 7765
评审: Jia LiLokesh KalekarAbhijit Kale
Abstract
Endothelial cells (ECs) sustain the self-renewal and regeneration of adult hematopoietic stem and progenitor cells (HSPCs) via deployment of EC-derived paracrine factors, termed as angiocrine factors. Generation of durable ex vivo vascular niche that maintains EC identity and preserves the angiocrine profile of organ of origin offers platforms for in vitro dissection of the mechanism by which angiocrine factors execute their instructive function for stem cell maintenance and tissue regeneration. This protocol describes detailed methods to isolate primary bone marrow ECs (BMECs), to subsequently transduce lentiviral vector carrying myristoylated-Akt1 into primary BMECs, and to use the Akt1-BMECs to expand engraftable murine HSPCs. The BMEC-HSPC co-culture system serves as bioreactor prototype to generate scalable populations of the blood and immune systems.
Keywords: Angiocrine (血管分泌因子)Background
Hematopoietic stem cells (HSCs) are multipotent adult stem cells that can self-renew to replenish themselves and differentiate into all the lineages of the blood and immune system. HSC transplantation offers the best therapeutic cure for diseases such as acute myeloid leukemia, and served as cellular platform to correct the mutation of genetic blood disease via gene targeting. There are several sources of hematopoietic stem cells, adult bone marrow-derived HSCs, cord blood-derived HSCs, and granulocyte-colony stimulating factor (GCSF)-mobilized HSCs. Compared with the bone marrow-derived HSCs, cord blood HSCs can tolerate more HLA mismatching, and have better anti-leukemia activities, and are more readily available. Unfortunately, the HSC transplantation is still a risky procedure to perform and transplant-related mortality is partially due to the leukemia relapse and/or the incidence of infections that took place during the recovery phase of HSC transplantation; all of which are attributable to the low stem cell numbers in the donor cord blood. Therefore, identifying cellular and molecular approaches that can help expand bona fide HSCs that maintain self-renewal activity is of pivotal translational significance.
Endothelial cells safeguard the self-renewal and regeneration of adult HSCs in the bone marrow via deploying endothelial-derived paracrine factors, termed as angiocrine factors, such as KitL, SDF-1, Jagged-1 and Jagged-2, etc. (Poulos et al., 2013; Mendelson and Frenette, 2014; Rafii et al., 2016, Asada et al., 2017). Rafii et al. have pioneered in the technology of isolating adult human bone marrow endothelial cells (BMECs) and performing co-culture experiments to expand HSPCs (Rafii et al., 1994). The short life span of human BMECs and human umbilical venous endothelial cells (HUVECs) were strategically (Zhang et al., 2004) overcome via overexpression of the adenoviral E4ORF1 gene. The resulting cells, termed as E4-HUVECs, maintain the angiocrine profiles of primary HUVECs and are able to support the self-renewal of long-term repopulating mouse HSPCs and human cord blood stem cells (Seandel et al., 2008; Butler et al., 2010). E4ORF1 executes such functions partially via activation of Akt1 signaling pathway. We have thus generated constitutively active Akt1 by adding a myristoylation sequence 5’ to the ORF sequence that helps target the Akt1 at the cell membrane to undergo phosphorylation. Transduction of myristoylated Akt1 into primary mouse BMECs maintains their EC identities and preserves the angiocrine profiles (Kobayashi et al., 2010). This approach is useful for in vitro assays to dissect the mechanism through which BMECs support the self-renewal and expansion of HSPCs (Poulos et al., 2013; Hadland et al., 2015; Poulos et al., 2015; Guo et al., 2017). With the concept of endothelial cell heterogeneity, Akt1-BMECs will prove to be a complementary approach for in vivo studies that highlight the instructive role of different vascular beds for the differentiation of subpopulation of the blood and immune system.
This protocol is divided into the following parts:
- FACSAria II sorting of primary BMECs.
- Dynabeads isolation of primary BMECs.
- Lentiviral transduction of primary BMECs.
- In vitro BMEC-HSPC coculture.
Materials and Reagents
- Falcon tube, 15 ml (Corning, catalog number: 352096)
- Falcon tubes, 50ml (Corning, catalog number: 352098)
- Falcon® 100 mm TC-treated Cell Culture Dish, 20/Pack, 200/Case, Sterile (Corning, catalog number: 353003)
- Low retention tube, 1.5 ml (Fisher Scientific, catalog number, FisherbrandTM, catalog number: 02-681-320)
- Kimwipes (KCWW, Kimberly-Clark, catalog number: 34120)
- Parafilm (Bermis, catalog number: PM996)
- Amicon Ultra-0.5 Centrifugal Filter Unit (Merck, catalog number: UFC503096)
- Sterile 40 μm nylon mesh (Corning, catalog number: 352340)
- 6-tube magnetic stand (Thermo Fisher Scientific, catalog number: AM10055)
- 24-well plate (Corning, catalog number: 353047)
- 12-well plate (Corning, catalog number: 353043)
- T75 flasks (Corning, catalog number: 353136)
- Bio-Spin® P-30 Gel Columns, Tris Buffer (Bio-Rad Laboratories, catalog number: 7326232)
- DynabeadsTM Sheep anti-rat IgG (Thermo Fisher Scientific, catalog number: 11035)
- DPBS, 1x without calcium and magnesium (Corning, catalog number: 21-031-CV)
- DMSO, dimethyl sulfoxide (Sigma-Aldrich, catalog number: D2650)
- Alexa FluorTM 647 NHS Ester (Succinimidyl Ester) (Thermo Fisher Scientific, catalog number: A20006)
- Isothesia (Isoflurane) solution (Henry Schein Animal Health, catalog number: 029405)
- Isoflurane chamber, EZ anesthesia
- Oxygen (Tech Air)
- Potassium chloride, KCl, BioXtra, ≥ 99.0% (Sigma-Aldrich, catalog number: P9333)
- Calcium chloride dihydrate, CaCl2•2H2O (Sigma-Aldrich, catalog number: C3306)
- Magnesium chloride, MgCl2, anhydrous, ≥ 98% (Sigma-Aldrich, catalog number: M8266)
- Sodium bicarbonate, NaHCO3 (Sigma-Aldrich, catalog number: S5761-500G)
- Bovine Serum Albumin, lyophilized powder, essentially IgG-free, low endotoxin, BioReagent, suitable for cell culture (Sigma-Aldrich, catalog number: A2058)
- HBSS buffer: Hank’s Balanced Salt Solution, 1x without Calcium, Magnesium and Phenol Red (Corning, catalog number: 21-022-CV)
- Lentiviral myristoylated-Akt1: Virus titer is measured by Lenti-X p24 Rapid Titer Kit (Takara Bio, Clontech, catalog number: 632200)
- Fibronectin (Sigma-Aldrich, catalog number: F1141-5MG) (working concentration is 1 μg/ml in PBS)
- Trypsin (Corning, catalog number: 25-052-CI)
- Collagenase (Roche Diagnostics, catalog number: 11088793001)
- Dispase (Roche Diagnostics, catalog number: 04942078001)
- Polybrene (Sigma-Aldrich, catalog number: H9268-5G)
- Direct lineage depletion kit (Miltenyi Biotec, catalog number: 130-110-470)
- Accutase cell detachment solution (Corning, catalog number: 25-058-CI)
- StemSpan SFEM (Stem Cell Technologies, catalog number: 09650)
- Knockout serum replacement (Thermo Fisher Scientific, catalog number: 10828028)
- Recombinant human SCF, or KitL (PeproTech, catalog number: 250-03)
- UltraPureTM 0.5 M EDTA, pH 8.0 (Thermo Fisher Scientific, catalog number: 15575020)
- DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride) (Thermo Fisher Scientific, catalog number: D1306)
- F-12 medium (Corning, Cellgro, catalog number: 10-080-CV)
- DMEM low-glucose medium (Corning, Cellgro, catalog number: 10-014-CV)
- Heat-inactivated FBS (Denville Scientific, catalog number: FB5001)
- Non-essential amino acid (Corning, Cellgro, catalog number: 25-025-CI)
- Penicillin/streptomycin/amphotericin (Corning, Cellgro, catalog number: 30-004-CI)
- 1 M HEPES (Corning, Cellgro, catalog number: 25-060-CI)
- Endothelial cell growth supplement (Alfa Aesar, catalog number: J64516)
- Heparin sodium 10 mg/ml (Sigma-Aldrich, catalog number: H3149-100KU)
- GlutaMAX (Thermo Fisher Scientific, catalog number: 35050061)
- Antibodies (Table 1)
- 8x stock concentration of collagenase/dispase solution (see Recipes)
- MACS buffer (see Recipes)
- Polybrene stock solution (see Recipes)
- EC complete medium (see Recipes)
Table 1. List of antibodies used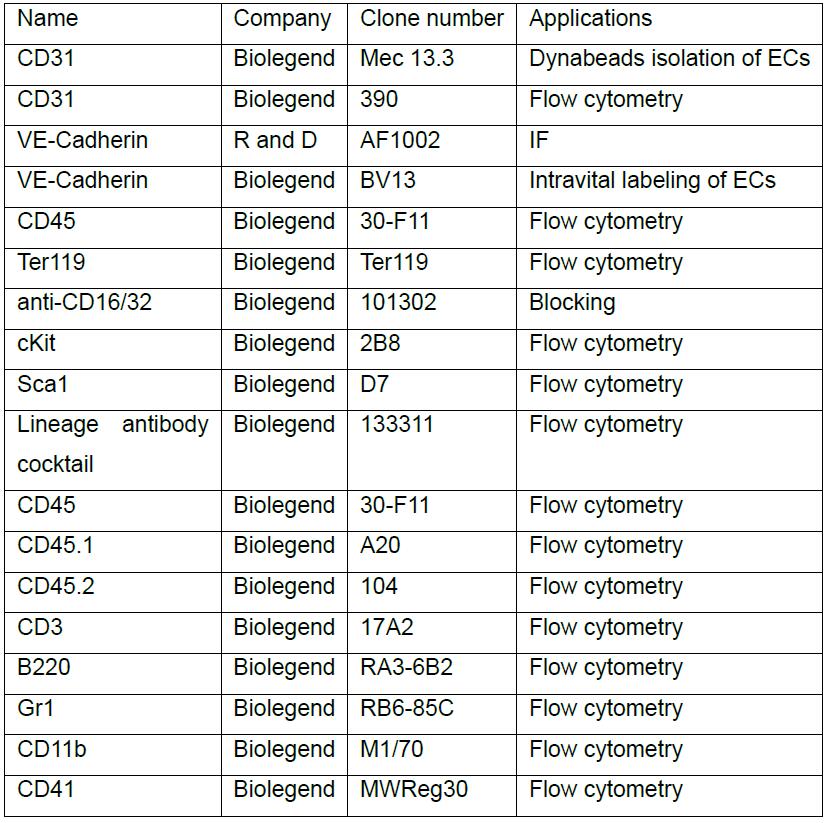
Equipment
- Pipettes, Denville Ultra EZpetteTM Pipette Starter Kit (Gray/Blue) (Denville Scientific, catalog number: P3960-SK)
- Scissors, Straight; Sharp-Blunt; 4.5" Length (Roboz Surgical Instrument, catalog number: RS-6800)
- Forceps, Student Grade Thumb Dressing Forceps 4.5" Serrated (Roboz Surgical Instrument, catalog number: 65-8100)
- Mortar and pestles. (VWR, catalog numbers: 89038-148 and 89038-164)
- 37 °C Orbital Shaker (Thermo Fisher Scientific, model: MaxQTM 4000)
- Centrifuge, SorvallTM LegendTM XT/XF Centrifuge Series (Thermo Fisher Scientific, model: SorvallTM LegendTM XT, catalog number: 75004505)
- FACS Aria II cell sorter (BD, model: FACSAria II)
- Laminar flow hood (The Baker Company, SterileGARD biological safety cabinets)
- NanoDrop Spectrometer (Thermo Fisher Scientific, model: NanoDropTM 1000, catalog number: ND-1000)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Guo, P. and Rafii, S. (2018). Generation of BMEC Lines and in vitro BMEC-HSPC Co-culture Assays. Bio-protocol 8(21): e3079. DOI: 10.21769/BioProtoc.3079.
分类
干细胞 > 成体干细胞 > 造血干细胞
发育生物学 > 细胞生长和命运决定 > 增殖
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link