- EN - English
- CN - 中文
Assessing Membrane Fluidity and Visualizing Fluid Membrane Domains in Bacteria Using Fluorescent Membrane Dyes
利用荧光膜染料评估膜流动性以及观察细菌中的流体膜结构区域
发布: 2018年10月20日第8卷第20期 DOI: 10.21769/BioProtoc.3063 浏览次数: 13686
评审: David CisnerosRon Saar DoverAgnieszka Zienkiewicz
Abstract
Membrane fluidity is a key parameter of bacterial membranes that undergoes quick adaptation in response to environmental challenges and has recently emerged as an important factor in the antibacterial mechanism of membrane-targeting antibiotics. The specific level of membrane fluidity is not uniform across the bacterial cell membrane. Rather, specialized microdomains associated with different cellular functions can exhibit fluidity values that significantly deviate from the average. Assessing changes in the overall membrane fluidity and formation of membrane microdomains is therefore pivotal to understand both the functional organization of the bacterial cell membrane as well as antibiotic mechanisms. Here we describe how two fluorescent membrane dyes, laurdan and DiIC12, can be employed to assess membrane fluidity in living bacteria. We focus on Bacillus subtilis, since this organism has been relatively well-studied with respect to membrane domains. However, we also describe how these assays can be adapted for other bacteria such as Staphylococcus aureus and Streptococcus pneumoniae.
Keywords: Membrane fluidity (膜流动性)Background
Bacterial membranes have long been viewed as homogenous lipid bilayers following the classical fluid mosaic membrane model. However, many studies have later shown that membranes are in fact highly organized structures and possess distinct domains that can be characterized by containing specific membrane proteins, the enrichment of certain lipid species, or by having a higher or lower membrane fluidity than the neighboring membrane areas (Lopez and Kolter, 2010; Bach and Bramkamp, 2013; Barák and Muchová, 2013; Strahl et al., 2014; Bramkamp and Lopez, 2015; Schneider et al., 2015; Müller et al., 2016). Two types of membrane domains that are characterized by a specific membrane fluidity are rigid lipid and regions of increased fluidity (RIFs). Both have been well-characterized in the Gram-positive model organism Bacillus subtilis (Bach and Bramkamp, 2013; Strahl et al., 2014). Evidence for specific membrane domains has also been found in the pathogenic bacteria Staphylococcus aureus (Garcia-Fernandez et al., 2017 and Weihs et al., 2018) and Streptococcus pneumoniae (Rosch and Caparon, 2005; Vega et al., 2013). Membrane fluidity appears to be a key factor that distinguishes these specific domains from the rest of the membrane. This is achieved by enrichment of fluidizing lipid species, i.e., branched-chain, unsaturated, and short-chain fatty acid-containing lipids. Bacteria can adapt their membrane fluidity by changing the ratios of branched/non-branched, unsaturated/saturated, and short-chain/long-chain fatty acids. In B. subtilis, lipid desaturation (rapid adaptation) and adjusting the ratio of iso and anteiso branched chain fatty acids (long-term adaptation) are the main mechanisms to adapt membrane fluidity in response to environmental challenges (Beranova et al., 2008; Kingston et al., 2011).
Recently, it has emerged that membrane fluidity and membrane domains of specific fluidity play a key role in the mechanism of action of membrane-active antibiotics (Epand and Epand, 2009; Müller et al., 2016; Saeloh et al., 2018). Therefore, it is crucial to be able to assess changes in both overall membrane fluidity and membrane domains when studying the in vivo activity of these compounds. We have recently established protocols for measuring membrane fluidity using two different fluorescent membrane probes, laurdan (Figure 1A) and DiIC12 (Figure 1B) (Strahl et al., 2014; Müller et al., 2016; Saeloh et al., 2018). Laurdan is a fluorescence probe that intercalates into the membrane bilayer and displays an emission wavelength shift depending on the amount of water molecules in the membrane (Parasassi and Gratton, 1995; Sanchez et al., 2007).
Laurdan generalized polarization (GP) can therefore be used as a reporter for head group density and fatty acyl spreading (Figure 1C). Laurdan fluorescence can be measured in a fluorescence plate reader and allows both end-point and kinetic measurements. It works well in 96-well plate format and allows relatively high throughput screening. Laurdan fluorescence can also be visualized under the microscope and used for single-cell analysis. It can further be employed for measuring the fluidity of liposomes, which can be important to distinguish direct and indirect antibiotic effects.
DiIC12 displays affinity for membrane areas of increased fluidity due to its short hydrocarbon tail (Baumgart et al., 2007; Zhao et al., 2013). Since fluid membranes are typically thinner (Reddy et al., 2012; Karabadzhak et al., 2018), it can be used as a reporter for membrane thickness and fluidity (Figure 1D). DiIC12 has been of key importance in the discovery of RIFs, fluid membrane microdomains that harbor the cell wall synthetic machinery in B. subtilis and E. coli (Strahl et al., 2014; Müller et al., 2016; Oswald et al., 2016). These domains are easily disturbed by membrane-active antibiotics and appear to play a key role in the mechanism of action of the last resort antibiotic daptomycin and the new antibiotic candidate rhodomyrtone (Müller et al., 2016; Saeloh et al., 2018). DiIC12 is ideally suited to visualize fluid membrane domains, whether natural or antibiotic-induced, in living cells. It can, however, also be used to stain liposomes. Together, laurdan and DiIC12 constitute a very useful assay combination to study both overall membrane fluidity and membrane domain formation in bacteria. We here describe the detailed protocols for measuring overall fluidity in batch culture and in liposomes as well as the distribution of membrane domains of different fluidity in single cells.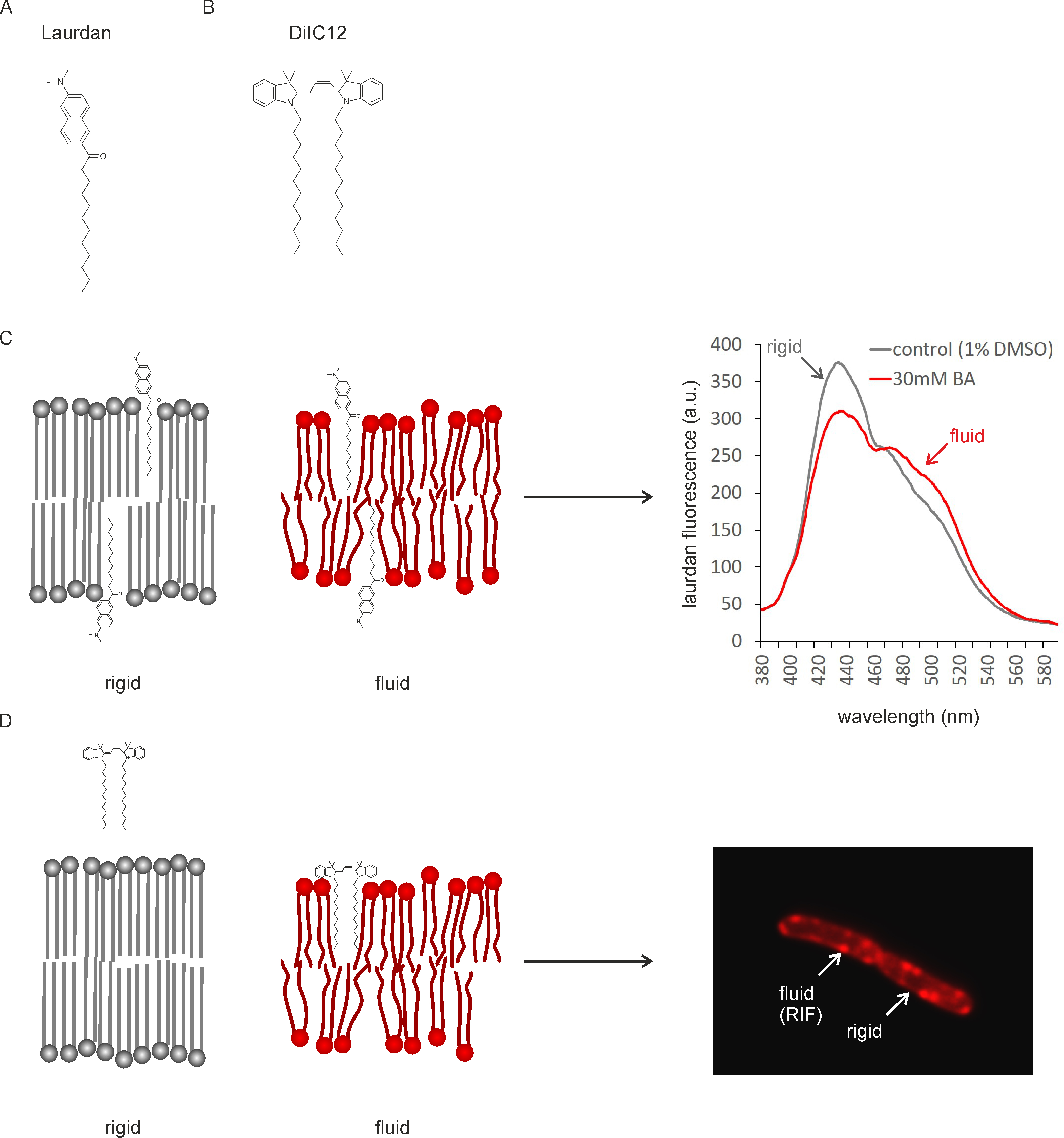
Figure 1. Mechanism of fluidity measurements with Laurdan and DiIC12. A. Structure of Laurdan; B. Structure of DiIC12; C. Laurdan inserts into membrane bilayers of different fluidity. Head group spreading and fatty acyl chain mobility determine the amount of water molecules around the laurdan molecule, which in turn causes a peak shift of the fluorescence probe. D. DiIC12 displays affinity for fluid membranes due to its short hydrocarbon tail, which is better accommodated in flexible, thin lipid bilayers. As a result, the dye accumulates in fluid membrane regions (RIFs). Note that due to better readability, dyes and lipids are not depicted to scale (dyes would be smaller).
Materials and Reagents
- 0.1-10 µl pipette tips (Gilson, catalog number: F161630)
- 0.1-2 µl pipette tips (Eppendorf, catalog number: 3120000011)
- 0.5-10 µl pipette tips (Eppendorf, catalog number: 3120000020)
- 0.2 µm Filtropur filters S (SARSTEDT, catalog number: 83.1826.001)
- 10 ml combitips for multistep pipette (Eppendorf, catalog number: 0030069.269)
- 100-1,000 µl pipette tips (Greiner, catalog number: 686290)
- 2 ml micro tubes (Greiner, catalog number: 623201)
- 2-20 µl pipette tips (Eppendorf, catalog number: 3120000038)
- 2-200 µl pipette tips (Greiner, catalog number: 739290)
- 5 ml combitips (Eppendorf, catalog number: 0030069.250)
- 50 ml Falcon tubes (SARSTEDT, catalog number: 62.547.254)
- 96-well plates, black flat-bottom (Screening Devices b.v., catalog number: 324002)
- Glass coverslips (Carl Roth GmbH, catalog number: NK75.1)
- Glass slides (Fisher-Emergo B.V., catalog number: 361000)
- Multi-spot glass slides (Hendley-Essex, catalog number: SM011, white)
- TetraSpeck microspheres, 0.2 µm, fluorescent blue/green/orange/dark red (Invitrogen via Thermo Fisher, catalog number: T7280)
- Black clear bottom microtiter plates (Greiner, catalog number: 675096)
- B. subtilis 168 (DSMZ, catalog number: 402)
- Agarose (Sphaero Q, catalog number: S103b)
- Ammonium iron (II) sulfate (Fe(II)NH4 citrate) (Sigma-Aldrich, catalog number: F5879)
- Ammonium sulfate ((NH4)2SO4) (Sigma-Aldrich, catalog number: A6387)
- Benzyl alcohol (Sigma-Aldrich, catalog number: 305197)
- Calcium chloride dihydrate (CaCl2•2H2O) (Merck, catalog number: 102382)
- Casein hydrolysate (casamino acids) (Duchefa, catalog number: C1301.0250)
- DiIC12 (1,1'-didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate) (Anaspec, catalog number: AS-84902)
- Dimethylformamide (DMF) (Sigma-Aldrich, catalog number: 227056)
- Dimethylsulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418)
- Disodiumhydrogenphosphate (Na2HPO4) (VWR, catalog number: 26932.290)
- E. coli polar lipid extract (Avanti Polar Lipids, catalog number: 100600P)
- Glucose (Duchefa Biochemie, catalog number: G0802)
- L-glutamic acid potassium salt (Sigma-Aldrich, catalog number: G1501)
- Gramicidin S (Sigma-Aldrich, catalog number: G0900)
Note: This product has been discontinued. The batch used in this study was kindly supplied by Marina Rautenbach, Stellenbosch University. - Hydrochloric acid (Merck, catalog number: 1003171000)
- Iron sulfate heptahydrate (FeSO4•7H2O) (Sigma-Aldrich, catalog number: 215422)
- Laurdan (6-Dodecanoyl-N,N-dimethyl-2-naphthylamine) (Sigma-Aldrich, catalog number: 40227)
- Magnesium sulfate heptahydrate (MgSO4•7H2O) (Roth, catalog number: T888.1)
- Manganese sulfate tetrahydrate (MnSO4•4H2O) (Fischer Scientific, catalog number: M/2250/53)
- MP196 (synthesized by solid-phase peptide synthesis according to Albada et al., 2012 and Sanchez et al., 2007); the batch used in this study was kindly supplied by Nils Metzler-Nolte, Ruhr University Bochum
- Potassium chloride (KCl) (VWR, catalog number: 26764.298)
- Potassiumdihydrogenphosphate (KH2PO4) (Merck, catalog number: 104873)
- Rhodomyrtone (purified form Rhodomyrtus tomentosa leaves according to Limsuwan et al. (Zhao et al., 2013); the batch used in this study was kindly supplied by Supayang Voravuthikunchai, Prince of Songkla University)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 1.06404.1000)
- Todd-Hewitt broth (Sigma-Aldrich, catalog number: T1438-500G)
- Tris (Merck, catalog number: 1083821000)
- Trisodium citrate dihydrate (Na3citrate•2H2O) (Merck, catalog number: 106448)
- Tryptone (Duchefa Biochemie, catalog number: T1332,1000MG)
- L-tryptophane (Sigma Aldrich, catalog number: T0254)
- Yeast extract (Duchefa Biochemie, catalog number: Y133)
- K2HPO4
- Agarose solution (see Recipes)
- BMM basic medium (see Recipes)
- BMM supplements (see Recipes)
- DiIC12 washing medium (see Recipes)
- Laurdan buffer (see Recipes)
- Lysogeny broth (LB) medium (see Recipes)
- SMM basic medium (see Recipes)
- SMM supplements (see Recipes)
Equipment
- 100-1,000 µl pipette (Eppendorf, catalog number: 3120.000062)
- 20-200 µl pipette (Eppendorf, catalog number: 3120000054)
- HeraCell 150 stove (Kendro, catalog number: 50075549B)
- Cy3 filter cube (Nikon, catalog number: MXU96213)
- DAPI filter cube (Nikon, catalog number: MBE41300)
- Laurdan custom filter cube composed of:
- C-FL filter block, frame only (Nikon, catalog number: MXA22030)
- FF01-360/23-25 excitation filter (Nikon, catalog number: MXR00637)
Important note: The same filter as in the DAPI filter cube. - Di02-R405-25x36 dichroic mirror (Nikon, catalog number: MXR00604)
Important note: The same dichroic mirror as in the DAPI filter cube. - FF01-535/5--25 emission filter (Nikon, catalog number: MXX99999)
- Multi step pipette (Eppendorf, catalog number: 022260201)
- Nikon Eclipse Ti inverted epi-fluorescence (wide-field) microscope (Nikon, catalog number: MEA53100) equipped with:
- CFI Plan Apochromat DM 100x NA 1.45 oil objective (Nikon, catalog number: MRD31905)
- Intensilight HG 130 W light source (MBF72665)
- C11440-22CU Hamamatsu ORCA Flash USB 3.0 camera (Nikon, catalog number: MHC11441)
- NIS elements AR software (Nikon, catalog number: MQS31000)
- Plate reader (BioTek Synergy Mx, catalog number: SMATBCL)
- Temperature-controlled microtube centrifuge (Eppendorf, model: 5424R, catalog number: 5404000010)
- Thermomixer (Eppendorf, catalog number: 5350000013)
- Water bath (GFL, catalog number: 5905985)
- Incubator Heraeus HERAcell (Kendro Laboratory Products, catalog number: 50042307)
- Autoclave (Sanyo, catalog number MLS-3780)
- Monochromators
- Spectrofluorometer Quantamaster 2000-4 (Photon Technology International)
Software
- ImageJ (https://imagej.nih.gov/ij/download.html)
- Fluorescence spectrophotometer software (PTI acquisition software FeliX32 version 1.2 Build 56)
- Microplate reader software Gen5 version 2.00 (Biotek)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Wenzel, M., Vischer, N. O. E., Strahl, H. and Hamoen, L. W. (2018). Assessing Membrane Fluidity and Visualizing Fluid Membrane Domains in Bacteria Using Fluorescent Membrane Dyes. Bio-protocol 8(20): e3063. DOI: 10.21769/BioProtoc.3063.
分类
微生物学 > 微生物细胞生物学 > 细胞染色
微生物学 > 抗微生物试验 > 抗细菌试验
细胞生物学 > 细胞染色 > 脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link