- EN - English
- CN - 中文
Optical Clearing and Index Matching of Tissue Samples for High-resolution Fluorescence Imaging Using SeeDB2
利用SeeD2培养基对组织样品光透明及折射率匹配以实现高分辨率成像
发布: 2018年10月20日第8卷第20期 DOI: 10.21769/BioProtoc.3046 浏览次数: 13193
评审: Shaarika SarasijaAnonymous reviewer(s)
Abstract
Tissue clearing techniques are useful for large-scale three-dimensional fluorescence imaging of thick tissues. However, high-resolution imaging deep inside tissues has been challenging, as it is extremely sensitive to light scattering and spherical aberrations. Here, we present a water-based optical clearing and mounting media, SeeDB2, which is designed for high numerical aperture (NA) objective lenses with oil or glycerol immersion. Using quick and simple soaking procedures, the refractive indices of samples can be matched either to that of immersion oil (1.52) or glycerol (1.46), thus minimizing light scattering and spherical aberrations. Fine morphology and various fluorescent proteins are highly preserved during the clearing and imaging process. Our method is useful for the three-dimensional fluorescence imaging of neuronal circuitry at synaptic resolution using confocal and super-resolution microscopy. SeeDB2 is also useful as a mounting media for the super-resolution imaging of fluorescent proteins.
Keywords: Tissue clearing (组织透明法)Background
Biological tissues are organized in 3D. In addition, many of important cellular machineries, e.g., synapses in neurons, are at sub-micron scale. Therefore, there have been increasing demands for a method for sub-micron-scale 3D imaging. Serial electron microscopy techniques (e.g., FIB-SEM or SBF-SEM) are promising, but they cannot make the best use of the genetic fluorescent labeling tools available in modern life science. To facilitate 3D imaging with fluorescence microscopy, a number of tissue clearing techniques have been developed in recent years (Richardson and Lichtman, 2015 and 2017). They are designed for large-scale 3D imaging, and some of them allow for whole-brain, and even whole-body-scale fluorescence imaging of fixed samples combined with confocal, two-photon, or light-sheet microscopy. However, many of them have not been fully optimized for high-resolution imaging.
In fluorescence microscopy, lateral resolution (d) is given as:
d = 0.61λ/NA
where λ is the wavelength of the light and NA represents the numerical aperture. The resolution improves as d decreases. Therefore, we need to use high NA objective lenses for high-resolution imaging.
NA is defined as:
NA = n sinα
where n is the refractive index (RI) of the immersion media, and α is the half angular aperture. Therefore, many of the high NA objective lenses are designed for oil (RI = 1.52) or glycerol (RI = 1.46) immersion for the best resolution. Previously, high NA objective lenses are intended to image thin sections or just the surface of samples. However, if we try to image deeper in the samples with these objective lenses, image quality will be easily impaired due to “spherical aberrations”. As RI of tissue samples are lower than that of immersion oil (RI = 1.52) and a glass coverslip (RI ~1.52), the excitation light will refract at the interface between the coverslip and samples, and it will no longer converge onto a small focal spot. This is known as spherical aberrations, reducing resolution and brightness in microscopy.
To minimize spherical aberrations, index matching of samples is crucial. However, many of the existing mounting media and clearing solutions have low RI, ranging from 1.33 (water) to 1.46 (glycerol-based mounting media). Even our previous clearing agent, SeeDB (RI = 1.49), did not reach RI 1.52 (Ke et al., 2013, 2014). 2,2'-thiodiethanol (TDE, RI = 1.52) has been previously proposed for index matching for oil-immersion objective lenses and has been widely used for synthetic fluorescent dyes (Staudt et al., 2007). However, a major drawback of TDE is that most of fluorescent proteins are totally quenched in TDE. To overcome this limitation, we developed a new tissue clearing agent, SeeDB2S, that has a high refractive index (RI = 1.52), but also highly preserves fluorescent proteins (Ke et al., 2016). We also formulated SeeDB2G (RI = 1.46) for glycerol-immersion objective lenses. As fluorescent proteins are better preserved than in commercialized mounting media, SeeDB2G/S are also useful as mounting media for high-resolution imaging. SeeDB2G/S is particularly powerful for high-resolution confocal microscopy and super-resolution microscopy of fluorescent proteins in tissues, sections, and cells.
Materials and Reagents
- 1.5 ml Eppendorf tube (Eppendorf, for 1 ml solution)
- 5 ml Eppendorf tube (Eppendorf, catalog number: 0030119401 ; optional for 3 ml solution, for thick brain slices or whole-mount samples)
- Paint brush (for thin brain slices, No. 1-2, see Figure 1)
- Silicone rubber sheet (translucent, 0.2 mm thick; e.g., AS ONE, catalog number: 6-9085-13 ; see Figure 1)
Note: Various thickness of silicone rubber sheets are available from Togawa Rubber (AS ONE), Professional Plastics, CS Hyde, etc., ranging from 0.1 mm to 8.0 mm. The thickness should match that of brain slices. - Glass slide (76 mm x 26 mm; MATSUNAMI Glass; Figure 1)
- Coverslips (18 x 18 mm, No 1.5H; e.g., Paul Marienfeld, catalog number: 0107032 or ZEISS, catalog number: 474030-9000-000 ; Figure 1)
Note: No 1.5H (170 ± 5 μm thick) is highly recommended for super-resolution imaging. - Sodium chloride (NaCl)
- Sodium hydrogen phosphate dodecahydrate (Na2HPO4•12H2O)
- Potassium chloride (KCl)
- Potassium dihydrogen phosphate (KH2PO4)
- 4% paraformaldehyde (PFA; e.g., NACALAI TESQUE, catalog number: 26126-25 ) in PBS
- 20% Saponin (NACALAI TESQUE, catalog number: 30502-42 ) in ddH2O with filter sterilization
Note: Different vendors prepare saponin from different species of plants. We strongly recommend NACALAI TESQUE. Brownish lots (often found in Sigma-Aldrich) should be avoided. - Low-melting point agarose (e.g., Thermo Fisher Scientific, catalog number: 16520100 )
- Omnipaque 350 (e.g., DAIICHI SANKYO, Omnipaque 350 Injection, 50 ml in 1 bottle; also available from GE healthcare)
- Histodenz (e.g., Sigma-Aldrich, catalog number: D2158 )
- Sodium azide (e.g., Sigma-Aldrich, catalog number: 13412 )
- 1 M stock of Tris-HCl, pH 7.6 (e.g., NACALAI TESQUE, catalog number: 35436-01 ), used to prepare Tris-EDTA buffer
- 0.5 M stock of EDTA, pH 8.0 (e.g., Dojindo, catalog number: 347-07481 ), used to prepare Tris-EDTA buffer
- Immersion media:
Glycerol (e.g., Type G Immersion Liquid, Leica Microsystems, catalog number: 11513910 ; Glycerine solution, Leica Microsystems, catalog number: 11513872 ) for SeeDB2G
Oil (Type F, Olympus, MOIL-30; also available from Leica, Zeiss, etc.) for SeeDB2S - (Optional) SeeDB2 Trial Kit (Wako Pure Chemical Industries, catalog number: 294-80701 )
- Phosphate-buffered saline (PBS) (see Recipes)
- Tris-EDTA buffer, pH 7.6 (see Recipes)
- Permeabilization solution (see Recipes)
- Solution 1 (see Recipes)
- Solution 2 (see Recipes)
- SeeDB2 solutions (see Recipes)
- SeeDB2G with saponin (clearing)
- SeeDB2G (mounting)
- SeeDB2S with saponin (clearing)
- SeeDB2S (mounting)
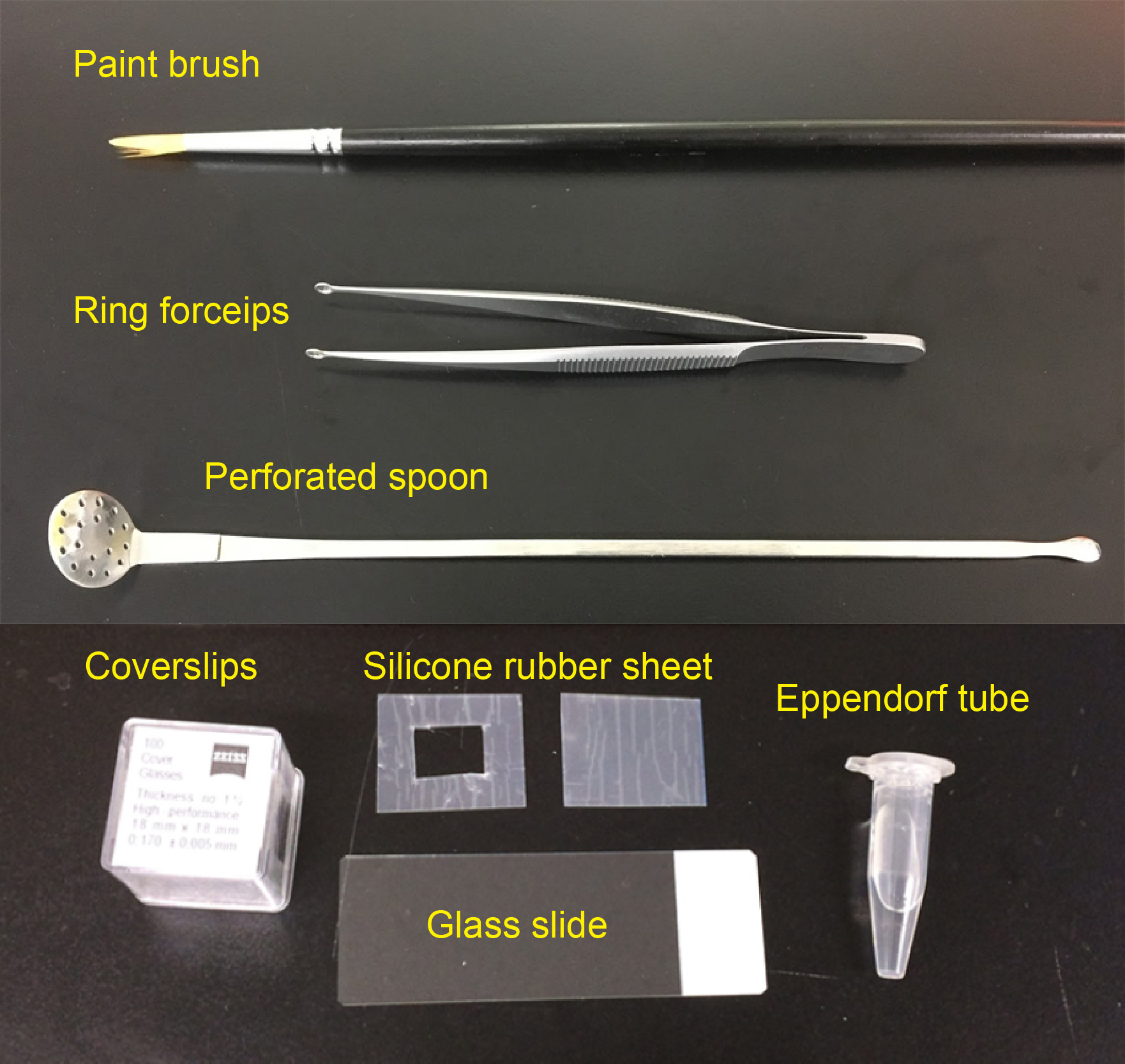
Figure 1. Materials required for preparing imaging chamber and slice mounting
Equipment
- Perforated spoon (optional for handling thick brain slices, custom-ordered, flat head, head diameter = 15 mm, see Figure 1 and Video 1)
- Ring forceps (optional for whole-mount samples, e.g., Natsume Seisakusho, NAPOX, catalog number: A-26 , see Figure 1)
- Vibratome (e.g., LinearSlicer, DOSAKA, model: PRO7N )
- Seesaw shaker (e.g., Bio Craft, model: BC-700 )
- Rotator (e.g., TAITEC, model: RT-30mini, catalog number: 0057154-000 )
- Fluorescence microscope
- Confocal microscope (e.g., Olympus, model: FV3000 ; Leica Microsystems, model: Leica TCS SP8 ; Nikon, model: A1+ )
- Super-resolution microscope (e.g., Zeiss, model: LSM 880 with Airyscan; Leica Microsystems, model: Leica TCS SP8 with HyVolution 2; Leica Microsystems, model: Leica TCS SP8 STED )
- Objective lenses
Examples: 63x oil-immersion (NA = 1.4, WD = 0.14 mm) (Leica Microsystems, model: HC PL APO 63x/1.4 Oil CS2, catalog number: 15506350 ); 100x oil-immersion (NA = 1.4, WD = 0.13 mm) (Leica Microsystems, model: HC PL APO 100x/1.4 Oil CS2, catalog number: 15506325 ); 63x glycerol-immersion (NA = 1.3, WD = 0.28 mm) (Leica, model: HCX PL APO 63x/1.30 GLYC CORR, catalog number: 11506193 ); 63x oil-immersion (NA = 1.4, WD = 0.19 mm) (Carl Zeiss, model: Plan-Apochroamt 63x/1.40 Oil DIC, catalog number: 440762-9904-000 )
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ke, M. and Imai, T. (2018). Optical Clearing and Index Matching of Tissue Samples for High-resolution Fluorescence Imaging Using SeeDB2. Bio-protocol 8(20): e3046. DOI: 10.21769/BioProtoc.3046.
分类
神经科学 > 神经解剖学和神经环路 > 荧光成像
发育生物学 > 形态建成 > 器官形成
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link