- EN - English
- CN - 中文
6-hydroxydopamine (6-OHDA) Oxidative Stress Assay for Observing Dopaminergic Neuron Loss in Caenorhabditis elegans
6-羟多巴胺(6-OHDA)氧化应激实验分析观察秀丽隐杆线虫多巴胺能神经元的损失
发布: 2018年09月20日第8卷第18期 DOI: 10.21769/BioProtoc.3025 浏览次数: 7503
评审: Khyati Hitesh ShahAnand Ramesh PatwardhanAnonymous reviewer(s)
Abstract
The nematode Caenorhabditis elegans is a powerful genetic model that can be used to investigate neuronal death. Research using C. elegans has been crucial to characterize cell death programmes that are conserved in mammals. Many neuronal signaling components, such as those mediating dopaminergic neurotransmission, are preserved as well. Dopaminergic neurons are progressively lost in Parkinson’s disease and an important risk factor to develop this disease appears to be oxidative stress, the increased occurrence of highly reactive oxygen species. Oxidative stress-induced dopaminergic neurodegeneration is mimicked in animal models by treatment with 6-hydroxydopamine (6-OHDA), a dopamine analog, which is specifically taken up into dopaminergic neurons. After exposing C. elegans to 6-OHDA, the loss of fluorescently labeled dopaminergic neurons can be easily monitored. An organisms’ sensitivity to oxidative stress is thought to be influenced by basal levels of intrinsic oxidative stress and the ability to counteract oxidative stress and oxidative stress-induced damage. The C. elegans ‘6-OHDA model’ led to the discovery of novel genes that are required to protect dopaminergic neurons and it has helped to determine the effects of conserved cell death and cell engulfment pathways in dopaminergic neurodegeneration. Here, we describe a simple protocol that allows for the easy detection of dopaminergic neuron loss after 6-OHDA treatment in C. elegans.
Keywords: C. elegans (秀丽隐杆线虫)Background
The gradual loss of dopaminergic neurons can be recapitulated in animal models following exposure to the oxidative stress-inducing drug 6-hydroxydopamine (6-OHDA) (for review Schober, 2004). In contrast to other neurodegenerative drugs such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), 6-OHDA is safer to handle as it does not pass the blood-brain-barrier. 6-OHDA is a hydroxylated dopamine analog, which is specifically taken up into dopaminergic neurons by the dopamine transporter and blocks complex I of the respiratory chain (Schober, 2004). The resulting formation of reactive oxygen species is thought to trigger 6-OHDA-induced neurodegeneration (Schober, 2004).
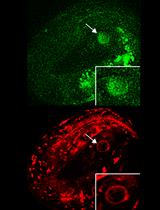
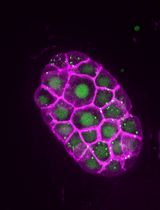
6-OHDA exposure of the nematode Caenorhabditis elegans leads to the selective loss of dopaminergic neurons (Nass et al., 2002) and components of dopaminergic neurotransmission are highly conserved compared to mammals (for review Nass et al., 2001). C. elegans hermaphrodites possess eight dopaminergic neurons: four CEP (cephalic sensilla) and two ADE (anterior deirids) dopaminergic neurons in the head, and two PDE (posterior deirids) dopaminergic neurons in the midbody (Sulston et al., 1975). These neurons can be specifically labeled by expression of a fluorescent protein driven by the promoter of the dat-1 dopamine transporter (Nass et al., 2002). The C. elegans 6-OHDA model can be used to understand how dopaminergic neurons maintain their integrity when subjected to oxidative stress.
The first published 6-OHDA intoxication protocol for C. elegans used high concentrations of 6-OHDA elicit dopaminergic neurodegeneration in wild-type animals (Nass et al., 2002). Mutation of the dopamine transporter dat-1, which is required for neuronal 6-OHDA uptake, was shown to confer 6-OHDA resistance (Nass et al., 2002). After 6-OHDA exposure during larval stages (L3 and L4), dopaminergic neurodegeneration was scored in a low-throughput manner by mounting adult animals on cover slide (Nass et al., 2002; Tucci et al., 2011). We adapted the protocol to screen for mutants that are hypersensitive to 6-OHDA exposure by using lower 6-OHDA concentrations that do not elicit neurodegeneration in wild-type animals. We expose synchronized L1 stage larvae in 96-well plates and score adults directly on agar plates, allowing for high-throughput screening. This approach led to the characterization of the tetraspanin gene tsp-17, the neuroligin-like gene glit-1 and the transthyretin-related gene ttr-33, all of which protect C. elegans dopaminergic neurons from 6-OHDA-induced neurodegeneration (Masoudi et al., 2014; Offenburger et al., 2018a and 2018b). The C. elegans 6-OHDA assay was further used to describe the roles of known stress response and cell death pathways in oxidative stress-induced dopaminergic neurodegeneration (Nass et al., 2002; Tóth et al., 2007; Offenburger et al., 2018a and 2018b).
The protocol can also be used for acute liquid exposure to other soluble compounds such as the oxidative stress-inducing drug paraquat (Offenburger et al., 2018a and 2018b). The procedures we describe here are generally useful to test if compounds influence dopaminergic neuron death.
Materials and Reagents
- Latex gloves
- Lab coat
- Platinum wire (e.g., CVS10 replacement platinum wire, 50 cm length x 0.2 mm diameter, Sigma-Aldrich, catalog number: EP1330 )
- High recovery filter pipette tips (e.g., Corning, Axygen® Maxymum Recovery®, catalog number: T-200-C-L-R-S )
- Bench surface protector sheets (GE Healthcare, Whatman®, catalog number: 2300-916 )
- Disposable spatulas (e.g., Disposable smartSpatula, LevGo, catalog number: 17251 )
- 1.5 ml screw cap tubes (sterile, graduated, conical, e.g., STARLAB, catalog number: E1415-2231 )
- 96-well plates (Tissue culture plates, round bottom, clear, sterile, TPP Techno Plastic Products, catalog number: 92097 )
- Only if filtering of L1 larval stages required: Nylon net filter, hydrophilic, 5 μm pore size (e.g., Merck, catalog number: NY0509050 with 90 mm diameter, or catalog number: NY0502500 with 25 mm diameter)
- 10 cm plastic Petri dishes
- Wet paper towel
- E. coli OP50 [Caenorhabditis Genetics Centre (CGC), University of Minnesota, Dept of GCD, 6-160 Jackson Hall, 321 Church Street S.E. Minneapolis, MN 55455, https://cgc.umn.edu/]
- Experimental control strains available at the C. elegans Genetics Centre (CGC), University of Minnesota, https://cgc.umn.edu/):
- TG2435 wild type-backcrossed BY200 derivate, vtIs1[pdat-1::gfp; rol-6] V (while pdat-1::gfp is always expressed, the penetrance of the roller phenotype is very low, only rarely detectable)
- TG2400 dat-1(ok157) III; vtIs1V
- TG4100 vtIs1 V; glit-1(gt1981) X
- TG2436 vtIs1 V; tsp-17(tm4995) X
- G4103 ttr-33(gt1983) V; vtIs1 V
- M9 buffer (He, 2011)
- Nematode growth medium (NGM) agar (He, 2011)
- LB (Luria-Bertani) liquid medium (see Cold Spring Harbor Protocols, 2006)
- 6-hydroxydopamine (6-OHDA) hydrochloride (Sigma-Aldrich, catalog number: H4381 ) (keep at -20 °C in the dark, prepare stock solution freshly)
- L-ascorbic acid (Sigma-Aldrich, catalog number: A5960 , ≥ 90%) (store at room temperature in the dark, wrap aliquots with aluminum foil)
- 200 mM ascorbic acid solution (freshly prepared, see Recipes)
- 10 mM 6-OHDA solution (freshly prepared, see Recipes)
Equipment
- Pipettes
- Metal inoculation loop
- Precision scales
- Temperature-controlled shaker (e.g., Eppendorf, model: ThermoMixer® R )
- Temperature-controlled incubator (20 °C)
- Fume hood
- Vortex Mixer (e.g., Scientific Industries, model: Vortex-Genie 2 )
- Ethanol burner (e.g., DWK Life Sciences, WheatonTM Alcohol Burner, catalog number: 237070 )
- Stereomicroscope with fluorescence (e.g., Leica)
Software
- R studio (version 1.0.44)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Offenburger, S. and Gartner, A. (2018). 6-hydroxydopamine (6-OHDA) Oxidative Stress Assay for Observing Dopaminergic Neuron Loss in Caenorhabditis elegans. Bio-protocol 8(18): e3025. DOI: 10.21769/BioProtoc.3025.
分类
神经科学 > 发育 > 形态建成
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link