- EN - English
- CN - 中文
GC-MS-Based Analysis of Methanol: Chloroform-extracted Fatty Acids from Plant Tissues
基于GC-MS法分析使用甲醇:氯仿从植物组织中提取的脂肪酸
发布: 2018年09月20日第8卷第18期 DOI: 10.21769/BioProtoc.3014 浏览次数: 15418
评审: Scott A M McAdamBedabrata SahaAli Parsaeimehr
Abstract
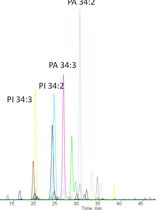
Fatty acids (FAs) are carboxylic acids with long aliphatic chains that may be straight, branched and saturated or unsaturated. Most of the naturally occurring plant FAs contains an even number of carbon (C4-C24). FAs are used in food and pharmacological industries due to their nutritional importance. In addition, FAs are considered as a promising alternative for the production of biodiesel from terrestrial plant biomass. To establish commercial applications, more reliable analytical methods are needed for the identification, quantification, and composition determination of FAs. Here, we describe a relatively rapid and sensitive method for the extraction, identification, and quantification of FAs from a small quantity of plant tissue. The method includes steps of lipid extraction, conversion of lipid to fatty acid methyl esters (FAMEs) by transmethylation, identification and quantification of FAMEs using gas chromatography-mass spectrometry (GC-MS). In this protocol, an internal standard is added prior to GC-MS analysis. The amount of each FA is calculated from its peak area relative to the peak area of the internal standard.
Keywords: Biodiesel (生物柴油)Background
Synthesis of fatty acids is important for the storage of metabolic energy. The increasing population and energy cost have emphasized the need to produce sustainable renewable fuels. The source of second-generation biofuels is non-food oilseed crops or lignocellulosic biomass mainly comprising of wastes of crop plants like perennial grasses including switchgrass, husks, straw and forest residue (Hadar, 2013). In this context, plants can serve as an excellent system to study fatty acid for nutraceutical and biodiesel aspects. Further, in biodiesel production, clean burn properties of the fuel are influenced by structural features of FAs including chain length and the degree of unsaturation (Knothe, 2005). Lignocellulosic biomass is a greener alternative to produce these products directly from cost-effective resources. FA profiling using GC-MS permits the normalization, annotation and quantification of a relatively wide variety of fatty acids in a single plant extract. The efficiency of lipid extraction depends on the polarity of the solvent. Polar lipids (such as glycolipids or phospholipids) are more soluble in polar solvents (such as alcohols) and non-polar lipids (such as triacylglycerols) are more soluble in non-polar solvents (such as chloroform). Thus, the total lipid extraction depends on the nature of the organic solvent. Bligh and Dyer (1959) established a method for total lipid extraction using the mixture of chloroform and methanol as a solvent. The total lipids were converted to fatty acid methyl ester by transmethylation (Carreau and Dubacq, 1978). In one study, it was observed that the solvent system of chloroform/methanol is very effective for lipid extraction (Sheng et al., 2011). Moreover, lipid recovery in terms of total lipid content, lipid class and FA composition of different microalgal extracts are also affected by different types of pretreatment and cell disruption techniques and solvents (Ryckebosch et al., 2012). The chloroform/methanol mixture was also found to be useful for the extraction of lipids from microalgae (Ryckebosch et al., 2012). Similarly, there are several other reports suggest that chloroform/methanol/phosphate buffer-based solvent system has a higher efficiency for the extraction of lipids from different microalgae and plants (Kumari et al., 2013; Mishra et al., 2015; Pandey et al., 2015; Patel et al., 2016). Chloroform/methanol mixture exhibits strong dissolving power for the entire range of polarity found in lipids. This mixture is also able to break up membranes and denature the proteins (Schreiner, 2006). The buffer helps to overcome the ionic adsorption effects of salt that may hinder lipid extraction in plant tissue. In most of the studies, methanolic NaOH and methanolic HCl were found to be appropriate derivatizing agents for the profiling of FAs in plants.
Based on this information, here we provide a detailed protocol for extraction of lipids, identification, and quantification of fatty acid methyl esters. We also provide a detailed formula to estimate the total saturated fatty acids (SFA), unsaturated fatty acids (MUFAs and PUFAs), unsaturation index (Poerschmann et al., 2004), degree of unsaturation (Ramos et al., 2009), atherogenic and thrombogenic indices (Simat et al., 2015). Analyses of FAs are done by GC-MS. This protocol provides a relatively rapid and reproducible method. Moreover, this method can be used to profile fatty acids from different types of plant tissues. The produced information can be useful in several contexts of nutraceutical, pharmacological and industrial purposes.
Materials and Reagents
- 5 ml, 2 ml and 1 ml glass pipettes (Borosil)
- Filter paper 40 (GE Healthcare, Whatman, catalog number: 1440-110 )
- 50 ml graduated centrifuge tube, PP (Tarsons, catalog number: 546041 )
- 2 ml GC vials and caps (Agilent Technologies, catalog number: 5190-2240 ) with 250 μl glass inserts (Agilent Technologies, catalog number: 5181-1270 )
- Pyrex culture tube, screw cap with PTFE liner (Corning, catalog number: 9826-13 )
- Plant tissue (Leaf, Stem, Root, and Fruit)
- Liquid nitrogen
- Nitrogen gas (> 99% Purity)
- HPLC-grade methanol (Merck, catalog number: 1060020500 )
- HPLC-grade chloroform (Merck, catalog number: 1024470500 )
- HPLC-grade water (Avantor Performance Materials, J.T. Baker, catalog number: 1.00577.2500 )
- HPLC-grade n-Hexane (Sigma-Aldrich, catalog number: 34859 )
- Potassium phosphate monobasic, ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: P0662 )
- Potassium phosphate dibasic, ACS reagent, ≥ 98% (Sigma-Aldrich, catalog number: P3786 )
- Sodium hydroxide (Sigma-Aldrich, catalog number: S8045 )
- F.A.M.E. Mix, C4-C24 (Sigma-Aldrich, catalog number: 18919-1AMP )
- Nonadecanoic acid (Sigma-Aldrich, catalog number: N5252 )
- Solvent extraction solution A (see Recipe 1)
- Solvent extraction solution B (see Recipe 2)
- Methanolic NaOH (see Recipe 3)
- Methanolic HCl (see Recipe 4)
- Internal standard (see Recipe 5)
Equipment
- 50 ml Flask (Borosil)
- Funnel (Borosil)
- Table-top centrifuge (Sigma Laborzentrifugen, model: 3-30KS )
- Sample concentrator (Hangzhou Allsheng Instruments, catalog number: MD200-2 )
- VacSeal liquid nitrogen dewar (Jencons-PLS, catalog number: 238-112 )
- Table-top spinix-vortex (Tarsons, catalog number: 3002 )
- Rotospin-Rotary mixer (Tarsons, catalog number: 3092 )
- Shaking water bath (JULABO, catalog number: SW23 )
- -20 °C New Brunswick premium freezers (Eppendorf)
- -80 °C New Brunswick premium freezers (Eppendorf)
- GC-MS (Shimadzu, model: GCMS-QP2010 ) coupled with mass spectrometer and equipped with an auto-sampler (AOC-5000) and flame ionization detection (FID)
- Balance (Sartorius, model: BSA224S-CW )
- The RTx-5MS capillary column (60 meters, 0.25 mm ID, and 0.5 μm df) (Rastek, catalog number: 13455 )
Software
- GC-MS solution Version 2.70, Post-run analysis
- Excel software (Microsoft office 2010)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Patel, M. K., Das, S. and Thakur, J. K. (2018). GC-MS-Based Analysis of Methanol: Chloroform-extracted Fatty Acids from Plant Tissues. Bio-protocol 8(18): e3014. DOI: 10.21769/BioProtoc.3014.
分类
植物科学 > 植物生物化学 > 脂质
生物化学 > 脂质 > 脂质测定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link