- EN - English
- CN - 中文
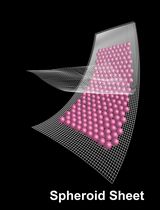
Generation of Human Mesenchymal Stem Cell 3D Spheroids Using Low-binding Plates
利用低结合培养板培养产生人间充质干细胞3D球状体
发布: 2018年08月20日第8卷第16期 DOI: 10.21769/BioProtoc.2968 浏览次数: 9760
评审: Giusy TornilloAlak MannaSurabhi Sonam
Abstract
The 3D culture of human mesenchymal stem cells (hMSCs) represents a more physiological environment than classical 2D culture and has been used to enhance the MSC secretome or extend cell survival after transplantation. Here we describe a simple and affordable method to generate 3D spheroids of hMSCs by seeding them at high density in a low-binding 96-well plate.
Spheroids of hMSCs cultured in low-binding 96-well plates can be used to study the basic biology of the cells and to generate conditioned media or spheroids to be used in transplantation therapeutic approaches. These MSCs or their secretome can be used as a regenerative therapy and for tissue repair across multiple disease areas, including neurodegeneration.
In comparison to other methods (hanging drop, use of gels or biomaterials, magnetic levitation, etc.), the method described here is simple and affordable with no need to use specialized equipment, expensive materials or complex reagents.
Background
Mesenchymal stem cells (MSCs) are an attractive candidate for the development of novel regenerative therapies for diseases such as stroke or amyotrophic lateral sclerosis (Chen et al., 2001; Bang et al., 2005; Boido et al., 2014). Their versatility makes the optimization and standardization of techniques essential to ensure MSC therapies can provide as much benefit as possible. One possible way to maximize the therapeutic potential (e.g., enhanced secretion of anti-inflammatory mediators) of MSCs is to culture them in 3D (Bartosh et al., 2010). Cells do not normally grow in monolayers in physiological conditions, therefore culturing them in 3D provides a more realistic environment, and increases secretion of certain factors such as vascular endothelial growth factor (VEGF) or granulocyte-colony stimulating factor (GCSF), amongst others (Caplan and Correa, 2011; Redondo-Castro et al., 2018). Some of these factors exert beneficial actions leading to an enhanced repair response (Torres-Espín et al., 2013; Kalladka and Muir, 2014) and by modulating the inflammatory component (Bernardo and Fibbe, 2013; Mathew et al., 2017).
Several methods have been developed to generate spheroids including magnetic levitation (Haisler et al., 2013); nanoparticles (Daquinag et al., 2013), hanging drop techniques (Bartosh et al., 2010; Murphy et al., 2014), suspension methods (Carpenedo et al., 2007) and hydrogels (Laschke et al., 2013; Tseng et al., 2017). Some of these methods, despite being effective, are time consuming or expensive as they require complex reagents or equipment (Cha et al., 2017). For this reason, we have been culturing spheroids using a very simple method (Redondo-Castro et al., 2018) that only requires a low-binding 96-well plate combined with a high-density suspension of cells.
With this method, we are able to obtain mature spheroids in a few days, with a very high rate of efficiency and reproducibility. Moreover, phenotypic characterization of spheroids shows that this method could be really useful for researchers developing cell therapies (either cell suspensions for transplants or generating cell-derived products such as conditioned media), as well as in other research fields.
Materials and Reagents
- Cell culture plasticware
- T75 and/or T25 flasks (Corning, catalog numbers: 430641U for T75 and 3056 for T25)
- Plates low cell binding, 96 wells, round bottom (Thermo Fisher Scientific, NuncTM, catalog number: 145399 )
- Centrifuge tubes (15 ml; 50 ml, Corning, catalog numbers: 430790 ; 430828 )
- Cryovials (STARLAB, catalog number: E3110-6122 )
- Plastic stripettes (5 ml; 10 ml; 25 ml, Corning, Costar®, catalog numbers: 4487 ; 4488 ; 4489 )
- Pipette tips (TipOne, STARLAB, catalog numbers: S1111-3700 ; S1111-1706 ; S1111-6701 )
- Non-adherent microfuge tubes (Eppendorf, catalog number: 0030108116 )
- Reagents
- DMEM low glucose (Sigma-Aldrich, catalog number: D6046 )
- Fetal bovine serum (FBS, Thermo Fisher Scientific, GibcoTM, catalog number: 10500064 )
- Gelatin, Analar (BDH, catalog number: 440454B )
- L-Glutamine, 200 mM (Sigma-Aldrich, catalog number: G7513 )
- MesenPRO RSTM Medium (Thermo Fisher Scientific, GibcoTM, catalog number: 12746012 )
- PBS, without calcium and magnesium (Sigma-Aldrich, catalog number: D8537 )
- Penicillin-streptomycin (P/S), 10,000 units penicillin and 10 mg streptomycin per ml (Sigma-Aldrich, catalog number: P0781 )
- Trypsin/EDTA 10x (Sigma-Aldrich, catalog number: T4174 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Trypan blue solution (Sigma-Aldrich, catalog number: T8154 , 0.4% [w/v] solution)
- Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 )
- Methanol (Fisher Scientific, CAS: 67-56-1)
- Fish skin gelatin (Sigma-Aldrich, catalog number: G7041 )
- Antibodies:
Mouse anti-fibrillin (used at 1/200, Merck, catalog number: MAB1919 )
Rabbit anti-fibronectin (used at 1/200, Sigma-Aldrich, catalog number: F3648 )
Donkey anti-mouse 680 nm (used at 1/400, LI-COR, catalog number: 926-68072 )
Donkey anti-rabbit 488 nM (used at 1/500, Thermo Fisher Scientific, InvitrogenTM, catalog number: R37118 ) - DAPI (used at 1/100,000,Thermo Fisher Scientific, InvitrogenTM, catalog number: D1306 )
- Gelatin solution (see Recipes)
- MesenPRO RSTM Medium (see Recipes)
- DMEM low glucose (see Recipes)
- Trypsin (see Recipes)
Equipment
- Water bath, 37 °C (Grant JB Nova)
- CO2 Incubator (Eppendorf, New BrunswickTM, model: Galaxy® 170 S )
- Glass hemocytometer (Brand)
- Laminar flow hood (ENVAIR, model: Envair Eco Safe Basic Plus )
- Inverted microscope (Olympus, model: CKX31 )
- Moticam 2300 camera coupled to Motic Images Plus 2.0 ML software (Motic, model: Moticam 2300 )
- Cell culture centrifuge (Sigma Laborzentrifugen, model: 3-16KL )
- Aspirator (dry vacuum pump/compressor, Welch Vacuum - Gardner Denver, model: 2511 )
- Autoclave (Prestige Medical, model: Classic 2100 Extended, catalog number: 210004UK )
Software
- Motic Images Plus 2.0 ML software, Motic®
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Redondo-Castro, E., Cunningham, C. J., Miller, J., Cain, S. A., Allan, S. M. and Pinteaux, E. (2018). Generation of Human Mesenchymal Stem Cell 3D Spheroids Using Low-binding Plates. Bio-protocol 8(16): e2968. DOI: 10.21769/BioProtoc.2968.
分类
干细胞 > 成体干细胞 > 间充质干细胞
细胞生物学 > 细胞分离和培养 > 3D细胞培养
细胞生物学 > 基于细胞的分析方法 > 非贴壁培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link