- EN - English
- CN - 中文
Measurement of TLR4 and CD14 Receptor Endocytosis Using Flow Cytometry
流式细胞术测定TLR4和CD14受体内吞作用
发布: 2018年07月20日第8卷第14期 DOI: 10.21769/BioProtoc.2926 浏览次数: 10520
评审: Ivan ZanoniMeenal SinhaBenoit Stijlemans

相关实验方案
在平板检测仪中使用高特异性荧光探针定量巨噬细胞细胞二价铁 (Fe2+) 含量
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
2024年02月05日 2198 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3522 阅读
Abstract
After recognizing extracellular bacterial lipopolysaccharide (LPS), the toll-like receptor 4 (TLR4)-CD14 signaling complex initiates two distinct signaling pathways–one from the plasma membrane and the other from the signaling endosomes (Kagan et al., 2008). Understanding the early stages of TLR4 signal transduction therefore requires a robust and quantitative method to measure LPS-triggered TLR4 and CD14 receptor endocytosis, one of the earliest events of LPS detection. Here, we describe a flow cytometry-based method that we used recently to study the role of the ion channel TRPM7 in TLR4 endocytosis (Schappe et al., 2018). The assay relies on stimulating the cells with LPS and measuring the cell surface levels of TLR4 (or CD14) at various time points using flow cytometry. Although we detail the method specifically for TLR4 and CD14 from murine bone marrow-derived macrophages, it can be readily adapted to evaluate receptor endocytosis in a variety of other signaling contexts.
Keywords: Toll-like receptor (Toll-类受体)Background
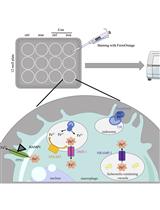
Innate immune cells, including macrophages and dendritic cells, employ a variety of pattern recognition receptors (PRRs) to survey their environments for danger- and pathogen-associated molecular patterns. Trafficking and signaling of PRRs from various subcellular compartments enables wider immune surveillance and has emerged as an important design principle of innate immunity (Brubaker et al., 2015). The detection of the bacterial endotoxin LPS is highly dependent on TLR4 and its co-receptor CD14. The endocytosis of the TLR4 complex requires CD14 and is essential for LPS-induced macrophage activation (Zanoni et al., 2011; Tan et al., 2015). Endocytosis of TLR4 is essential to activate secondary signaling complexes at the newly-formed ‘signaling endosome,’ which promotes interferon regulatory factor 3-dependent transcription through the signaling adaptor TIR-domain containing adapter-inducing interferon-β (TRIF) (Kagan et al., 2008). TLR4 endocytosis has been observed in macrophages, dendritic cells, and epithelial cells (Roy et al., 2014). Understanding the underlying mechanisms of this critical step in macrophage activation requires a robust and quantitative method to measure LPS-triggered TLR4 endocytosis. Here, we describe a version of a flow cytometry-based method that was initially reported by Kagan and colleagues (Kagan et al., 2008), and used by others, to monitor TLR4 endocytosis. We have used the method recently to study the role of transient receptor potential melastatin-like 7 (TRPM7), an ion channel, in TLR4 endocytosis (Schappe et al., 2018). The experimental logic of this method relies on measuring the loss of TLR4 and CD14 staining at the cell surface after LPS treatment. We stain LPS-treated cells with an anti-TLR4 (or anti-CD14) fluorophore-conjugated antibody without permeabilization. The fluorescence intensity acquired using flow cytometry reports the relative quantity of receptor resident in the plasma membrane (Figure 1). Although specific for TLR4 and CD14, the assay can be readily adapted to evaluate receptor endocytosis in a variety of other signaling contexts.
Figure 1. Schematic of TLR4 and CD14 endocytosis protocol. Experimental workflow described in protocol “Procedure”.
Materials and Reagents
- Materials
- Pipette tips
- 5 ml round, disposable round-bottom tube (FACS Tube) (Corning, Falcon®, catalog number: 352052 )
- Aluminum foil (Genesee Scientific, catalog number: 88-101 )
- 0.2 μm bottle filter (Thermo Fisher Scientific, NalgeneTM, catalog number: 566-0020 )
- 6-well non-treated culture plates (Corning, catalog number: 3736 )
- Sterile cell scrapers (Fisher Scientific, FisherbrandTM, catalog number: 08-100-240 )
- Sterile individually packaged serological pipette (10 ml) (Greiner Bio One International, catalog number: 607160 )
- Sterile individually packaged serological pipette (5 ml) (Greiner Bio One International, catalog number: 606160 )
- 1.7 ml microfuge Eppendorf tubes (Genesee Scientific, Olympus Plastics, catalog number: 24-281 )
- NuncTM TripleFlaskTM Treated Cell Culture Flasks (Thermo Fisher Scientific, catalog number: 132867 )
- Falcon® 50 ml Conical Centrifuge Tube (Corning, catalog number: 352098 )
- Pipette tips
- Cell line
- L-929 cells (ATCC, catalog number: CCL-1 )
- L-929 cells (ATCC, catalog number: CCL-1 )
- Reagents
- LPS EB-Ultrapure (lipopolysaccharide from E. coli O111:B4, InvivoGen, catalog number: tlrl-3pelps )
- PBS (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- Mouse TruStain fcXTM (anti-CD16/32) (BioLegend, catalog number: 101320 )
- TLR4 [anti-mouse CD284] (PE) (clone: SA15-21; isotype: Rat IgG2a, κ) (BioLegend, catalog number: 145404 )
- CD14 [anti-mouse] (APC) (clone: Sa2-8; isotype: Rat IgG2a, κ) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 17-0141-81 )
- RPMI 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 11875093 )
- Fetal bovine serum (heat-inactivated), certified, USA origin (Thermo Fisher Scientific, GibcoTM, catalog number: 10082147 )
- Trypan blue (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- HBSS, no calcium, no magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 14170112 )
- BSA (Bovine serum albumin) (Roche Molecular Systems, catalog number: 3116956001 )
- DMEM, high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 11965092 )
- BMDM Media (see Recipes)
- Culture Media (see Recipes)
- Treatment Media (see Recipes)
- FACS Buffer (see Recipes)
- L929-conditioned media (see Recipes)
- LPS EB-Ultrapure (lipopolysaccharide from E. coli O111:B4, InvivoGen, catalog number: tlrl-3pelps )
Equipment
- TC20 Automated cell counter (Bio-Rad Laboratories, catalog number: 1450102 )
- Pipet-aid Pipette Controller (Drummond Scientific, catalog number: 4-000-101 )
- 4 °C Cold Room
- 4 °C Benchtop centrifuge
- 37 °C Cell Culture Incubator with CO2 control
- Sterile cell culture hood
- Flow Cytometer (BD, model: FACSCantoTM II , or equivalent)
Software
- GraphPad Prism 7 (Graph Pad Software; La Jolla, CA USA)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Schappe, M. S. and Desai, B. N. (2018). Measurement of TLR4 and CD14 Receptor Endocytosis Using Flow Cytometry. Bio-protocol 8(14): e2926. DOI: 10.21769/BioProtoc.2926.
分类
免疫学 > 免疫细胞功能 > 巨噬细胞
细胞生物学 > 基于细胞的分析方法 > 细胞吞排作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link