- EN - English
- CN - 中文
Brain Tissue Culture of Per2::Luciferase Transgenic Mice for ex vivo Bioluminescence
用于离体生物发光 的Per2::萤光素酶转基因小鼠脑组织的培养
发布: 2018年07月05日第8卷第13期 DOI: 10.21769/BioProtoc.2917 浏览次数: 7264
评审: Oneil G. BhalalaKarthik KrishnamurthyAnonymous reviewer(s)
Abstract
In circadian research, it is essential to be able to track a biological rhythm for several days with the minimum perturbation for the organisms or tissues. The use of transgenic mice lines, in which the luciferase reporter is coupled to a molecular clock protein (here PERIOD2), gives us the opportunity to follow the circadian activity in different tissues or even single clock cells for days without manipulation. This method creates sections using a mouse brain matrix, which allows us to obtain several brain samples quickly at a single time point.
Keywords: Bioluminescence (生物发光)Background
Circadian rhythms are behavioral or molecular changes that follow roughly 24 h-cycles and are sustained without any external cue. In mammals, locomotor activity, body temperature and hormone release are examples of circadian rhythms which are under the control of the suprachiasmatic nucleus (SCN) clock located in the hypothalamus. The ability of the SCN cells to keep an endogenous rhythm is due to a molecular machinery composed by the positive and negative loops of the expression of clock genes: firstly, CLOCK and BMAL1 proteins heterodimerize to activate the transcription of different genes through E-box sites on the promoter which is on genes like period (Per1-3) and cryptochrome (Cry1-2; Takahashi et al., 2008). Then, the proteins of PERIOD and CRYPTOCHROME heterodimerize and enter back to the nucleus to prevent BMAL1 binding to the E-Box. Hence, PERIOD and CRYPTOCHROME inhibit their own transcription (Takahashi et al., 2008). A second loop is made by retinoid-related orphan receptors (ROR) and Rev-Erb: the ROR proteins activate Bmal1 gene while REV-ERB proteins inhibit it via ROR-response element in the Bmal1 promoter. All this mechanism oscillates within a 24 h-period (Takahashi et al., 2008).
In circadian research, it is important to follow rhythmic activity in the whole organism or tissues around the 24 h. For that, it is necessary to get tissues or samples at different time points to model the oscillations of gene, protein expression or hormonal release. However, these methods require more than one animal per time point, and therefore it requires a lot of animals to get a complete and significant circadian oscillation.
In 2000, Yamazaki et al., created a transgenic rat line to solve this problem. They inserted a vector containing the luciferase gene from the firefly under the control of Per1 promoter. Since the 80’s, the luciferase has been used as ATP, gene or protein reporter. This 61-kDa enzyme has the particularity to release photon by oxidation of its substrate and in the presence of ATP, Mg2+ and oxygen (Gould and Subramani, 1988). The beetle luciferase has the advantage to be a single protein with no post-translational modification; its catalytic area is ready-to-use after its translation and minimal auto-fluorescence throughout recording (Bioluminescent Reporters [Reference #1]).
Although Per1-luciferase rat is an advance in circadian field, it does not allow us to follow the endogenous clock gene expression, but rather the endogenous activity of the heterodimer CLOCK-BMAL1. In 2004, Takahashi lab created the transgenic mouse line in which the open reading frame (ORF) of the luciferase is fused to the end of the Per2 gene (Yoo et al., 2004). All cells expressing the PER2 protein are also able to produce yellow-green light (~560 nm) in the absence of external light source if they have access to the luciferin: the consumable substrate. The bioluminescence produced by these cells permits to follow the circadian clock activity of the same individual for several days, and even weeks.
The principal aim of this technique is to dissect the brain region of interest of several animals at a single time point. For that, we used a mouse brain matrix that requires less tissue preparation and slices faster than a vibratome. However, the disadvantage of this technique is the loss of thickness precision (~500 µm). The vibratome cuts thinner and more precise tissue slices, but all the related procedure requires time. The advantage of the use of the matrix is to have few steps to work rapidly on the area of interest.
Materials and Reagents
- Gloves
- Carbon steel scalpel No. 24 (Swann-Morton, catalog number: 0211 )
- Double edge stainless steel razor blade (Electron Microscopy Sciences, catalog number: 72000 )
- NuncTM cell culture/Petri dishes (35 mm, Thermo Fisher Scientific, catalog number: 150318 )
- 10 ml syringe (Terumo Medical, catalog number: SS-10ES )
- Corning® vacuum filter system 500 ml, sterile, pore size: 0.22 µm (Corning, catalog number: 431097 )
- Sterile sampling pot: aseptic 40 ml polypropylene straight container with screw cap (Dominique DUTSCHER, Corning GOSSELINTM, catalog number: 688252 )
- Falcon Corning® 15 ml PP Centrifuge Tubes (Corning, catalog number: 430791 )
- Falcon Corning® 50 ml PP Centrifuge Tubes (Corning, catalog number: 430829 )
- FisherbrandTM SureOneTM 0.1-10 µl aerosol barrier pipette tips (Fisher Scientific, catalog number: 11903466 )
- FisherbrandTM SureOneTM 20-200 µl aerosol barrier pipette tips (Fisher Scientific, catalog number: 11963466 )
- FisherbrandTM SureOneTM 100-1,000 µl aerosol barrier pipette tips (Fisher Scientific, catalog number: 11973466 )
- Costar® 5 ml Stripette® serological pipets, sterile (Corning, catalog number: 4487 )
- Costar® 10 ml Stripette® serological pipets, sterile (Corning, catalog number: 4488 )
- Costar® 25 ml Stripette® serological pipets, sterile (Corning, catalog number: 4489 )
- Axygen® 0.2 ml Thin Wall PCR Tubes with Flat Cap (Corning, catalog number: PCR-02-C )
- Axygen® 0.6 ml Maxy Clear SnaplockMicrocentrifuge Tube (Corning, catalog number: MCT-060-C )
- Millicell® cell culture insert (Merck, catalog number: PICMORG50 )
- Per2::Luciferase homozygote knock-in (KI) Musmusculus (Per2tm1Jt)
Note: Mice were initially from Jackson Laboratories. Generally, we used young-adult (2-6 months old) mice, males as well as females, from our own breeding colony (Chronobiotron platform, UMS-3415 in Strasbourg). Aside from specific protocols, mice were housed in groups–of 3 or 4 individuals with food and water available ad libitum– in light-proof ventilated rooms, under 12 h white light and 12 h dim red light (< 5 lux at cage level) cycle (LD12:12; lights on at 7:00 A.M.). - Antibiotic (penicillin-streptomycin 10,000 U/ml; 10,000 mg/ml, Sigma-Aldrich, catalog number: P4333 ), stored at -20 °C
- B27 (Thermo Fisher Scientific, catalog number: 17504044 ), stored at -20 °C
- Hank’s balanced salt solution with red phenol (HBSS; 10x, Sigma-Aldrich, catalog number: H1641 ) stored at room temperature
- HEPES (Sigma-Aldrich, catalog number: H0887 ), stored at 4 °C
- Sodium bicarbonate (Sigma-Aldrich, catalog number: S8761 ), stored at 4 °C
- Bactericide/fungicide: ANIOSYME DD1 (Laboratoires ANIOS, Lille-Hellemmes, France)
- Commercial chlorine in tablets to make 10x bleach
- Dulbecco's modified Eagle's medium 10x (DMEM) with low glucose without red phenol (Sigma-Aldrich, catalog number: D2902 ), stored at 4 °C
- D (+) Glucose (Sigma-Aldrich, catalog number: G7021 ), stored at room temperature
- Beetle luciferin (Promega, catalog number: E1602 ), stored at -80 °C
- High vacuum grease (Dow corning®, Wiesbaden, Germany), stored at room temperature
- MilliQ Water
- EtOH 70%
- 0.1 M luciferin (see Recipes), stored at -20 °C
- HBSS 1x (see Recipes), stored at 4 °C
- DMEM 1x (see Recipes), stored at 4 °C
- Cleaning solution for tools (see Recipes)
Equipment
Note: All items without reference can be ordered from any qualified company.
- Stainless steel mouse brain matrix (Adult Mouse Brain Slicer Matrix, Zivic Instruments, catalog number: BSMAS005-1 )
Note: Other brain matrixes (for hamsters or rats) exist. - Stainless steel tweezers, fine tips, straight
Note: To have a better sight of the dissection tools, see Figure 1.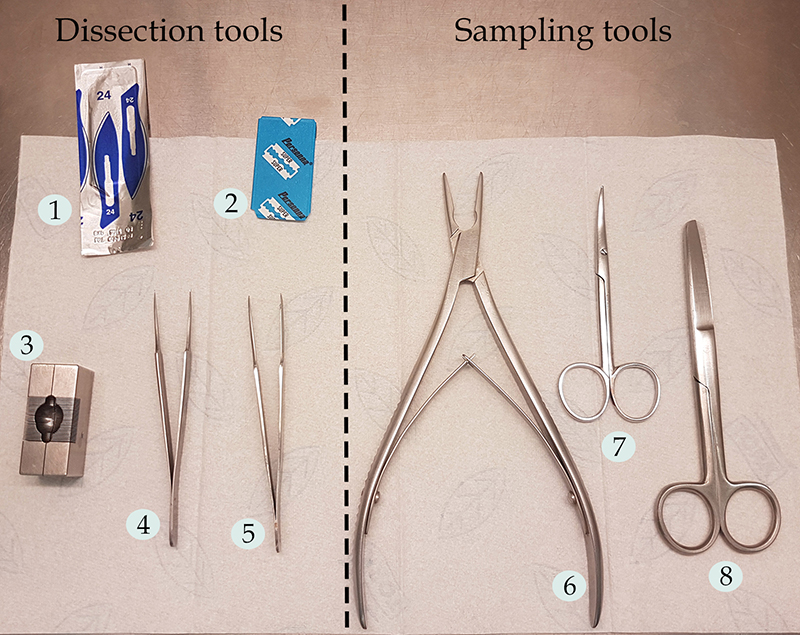
Figure 1. Surgery instruments used during the protocol. 1) Carbon steel scalpel No. 24; 2) Double edge stainless steel razor blade; 3) Stainless steel mouse brain matrix; 4-5) Stainless steel tweezers, fine tips, straight; 6) Friedman bone rongeur; 7) Curved Scissors, fine tips; 8) Stainless steel operating scissors, straight. - Stainless steel operating scissors, straight
- Curved Scissors, fine tips
- Friedman bone rongeur
- Vacuum pump (KNF, catalog number: N86KN.18 )
- Red light lamp (around 620-650 nm)
- Fiber optic light source (SCHOTT, catalog number: KL 1500LCD )
- Stereo Microscope (Nikon, catalog number: 536087 )
- Sterilized BRAND® Petri dish, glass, size 100 mm x 15 mm (BRAND, catalog number: 455742 )
- 1 L sterilized bottle
- 500 ml sterilized bottle
- 100 ml sterilized measuring cylinder
- 1 L sterilized measuring cylinder
- 1 L sterilized beaker
- Magnet
- Magnetic plate (size: big enough for 1 L beaker; strength: enough to dissolve powder into a liquid)
- P10 pipetman® classic (Gilson, catalog number: F144802 )
- P20 pipetman® classic (Gilson, catalog number: F123600 )
- P200 pipetman® classic (Gilson, catalog number: F123601 )
- P1000 pipetman® classic (Gilson, catalog number: F123602 )
- Pipetboy acu 2 (INTEGRA Biosciences, catalog number: 155019 )
- Milli-Q® water system
- Ice maker
- Fridge (4 °C)
- Fume hood (horizontal flow)
- Fume hood (vertical flow)
- Autoclave
- Photo-counting apparatus as LumiCycle 32 (Actimetrics, Wilmette, IL, USA) inside 36.5 °C incubator
- 37 °C incubator
Note: Petri dishes will be sealed. We do not need 5% CO2; an incubator that keeps only medium or dishes at 37 °C is enough.
Software
- LumiCycle Analysis for data extraction
- Table software like Microsoft Office Excel for data extraction
- SigmaPlot for statistics
- GraphPad for graphs
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Salaberry, N. L. and Mendoza, J. (2018). Brain Tissue Culture of Per2::Luciferase Transgenic Mice for ex vivo Bioluminescence. Bio-protocol 8(13): e2917. DOI: 10.21769/BioProtoc.2917.
分类
神经科学 > 细胞机理 > 组织分离与培养
细胞生物学 > 组织分析 > 组织记录
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link