- EN - English
- CN - 中文
Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism
测定培养细胞中的消氧率(OCR)和细胞外酸化率(ECAR)以评估能量代谢
发布: 2018年05月20日第8卷第10期 DOI: 10.21769/BioProtoc.2850 浏览次数: 75937
评审: Amriti Rajender LullaAnonymous reviewer(s)
Abstract
Mammalian cells generate ATP by mitochondrial (oxidative phosphorylation) and non-mitochondrial (glycolysis) metabolism. Cancer cells are known to reprogram their metabolism using different strategies to meet energetic and anabolic needs (Koppenol et al., 2011; Zheng, 2012). Additionally, each cancer tissue has its own individual metabolic features. Mitochondria not only play a key role in energy metabolism but also in cell cycle regulation of cells. Therefore, mitochondria have emerged as a potential target for anticancer therapy since they are structurally and functionally different from their non-cancerous counterparts (D'Souza et al., 2011). We detail a protocol for measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) measurements in living cells, utilizing the Seahorse XF24 Extracellular Flux Analyzer (Figure 1). The Seahorse XF24 Extracellular Flux Analyzer continuously measures oxygen concentration and proton flux in the cell supernatant over time (Wu et al., 2007). These measurements are converted in OCR and ECAR values and enable a direct quantification of mitochondrial respiration and glycolysis. With this protocol, we sought to assess basal mitochondrial function and mitochondrial stress of three different cancer cell lines in response to the cytotoxic test lead compound mensacarcin in order to investigate its mechanism of action. Cells were plated in XF24 cell culture plates and maintained for 24 h. Prior to analysis, the culture media was replaced with unbuffered DMEM pH 7.4 and cells were then allowed to equilibrate in a non-CO2 incubator immediately before metabolic flux analysis using the Seahorse XF to allow for precise measurements of Milli-pH unit changes. OCR and ECAR were measured under basal conditions and after injection of compounds through drug injection ports. With the described protocol we assess the basic energy metabolism profiles of the three cell lines as well as key parameters of mitochondrial function in response to our test compound and by sequential addition of mitochondria perturbing agents oligomycin, FCCP and rotenone/antimycin A.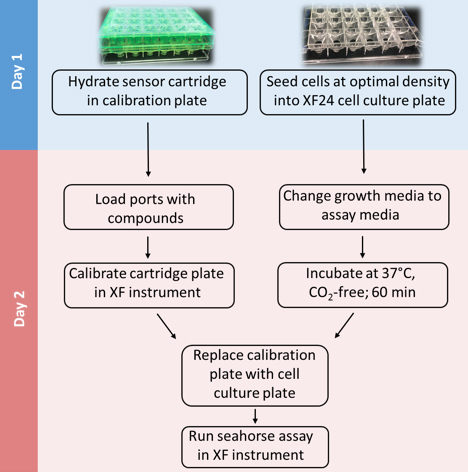
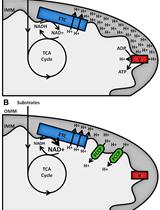
Figure 1. Overview of seahorse experiment
Background
Natural products are small molecules that are isolated from natural sources. Over the last century, these molecules have been instrumental in treating human diseases, especially inspired chemotherapeutics. Metabolites like taxol, vincristine, and doxorubicin have revolutionized how we treat malign cancers and other natural products, for example rapamycin, oligomycin, and bafilomycin, are used as molecular probes and enable molecular studies of biochemical and cellular processes in the laboratory. While studying the mechanism of action of the cytotoxic natural product mensacarcin, we found that a fluorescently labeled mensacarcin probe localizes to a great extent in mitochondria (Plitzko et al., 2017). To investigate if mensacarcin’s cytotoxic properties might be derived from interference with mitochondrial function, we sought to examine mensacarcin’s effects on cellular bioenergetics. Using a Seahorse Extracellular Flux Analyzer, we monitored cellular oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) in real time as measures of mitochondrial respiration and glycolysis, respectively (Wu et al., 2007; Serill et al., 2015). The Seahorse XF24 Extracellular Flux Analyzer allows continuous direct quantification of mitochondrial respiration and glycolysis of living cells. The instrument uses a sensor cartridge in a 24-well plate format with each sensor being equipped with two embedded fluorophores: one which is quenched by oxygen (O2) and the other that is sensitive to change in pH. During measurements, the sensor cartridge is lowered 200 µm above the cell monolayer, forming a micro-chamber of about 2 µl. The Seahorse instrument contains fiber optic bundles that emit light, excite the fluorophores, and then measures the change in the fluorophore’s emission. The very small test volume formed by the transient micro chamber allows for sensitive, precise, and nondestructive measurements of parameters in real time. Changes in oxygen concentration and pH are automatically calculated and reported as Oxygen Consumption Rate (OCR) and Extra Cellular Acidification Rate (ECAR). Once a measurement is completed, the sensors lift which allows the larger medium volume above to mix with the medium in the transient micro chamber, restoring values to baseline. The sensor cartridge contains ports that allow sequential addition of up to four compounds per well during the assay measurements.
With the described protocol we assessed the energy metabolism of three cell lines (HCT-116, SK-Mel-28, and SK-Mel-5) (Figure 6). Addition of mensacarcin was found to have pronounced effect on the basal OCR of melanoma cells and no increasing effect on ECAR. An increase in glycolysis is often observed as a compensatory response. Mitochondria are essential for the energy metabolism of cells and have a key role in apoptotic cell death. Alteration of the mitochondrial respiration or the equilibrium between the pro-apoptotic and anti-apoptotic proteins can induce mitochondrial failure. To gain insights into the induced mitochondrial impairment in melanoma cells, we assessed key parameters of mitochondrial respiration by consecutively exposing cells to well described mitochondria perturbing reagents. Following addition of our test compound mensacarcin, we sequentially added oligomycin, FCCP, and lastly rotenone and antimycin A (Figure 5). Oligomycin inhibits ATP synthase and reduces OCR, FCCP uncouples oxygen consumption from ATP production and raises OCR to a maximal value, and antimycin A and rotenone target the electron transport chain and reduce OCR to a minimal value. The mitochondria stress test protocol provides information on basal respiration, ATP-linked respiration, proton leak, maximal respiration capacity, and non-mitochondrial respiration of cells. Therefore, this assay can be used to provide insight on the mechanism of action of compounds that directly target mitochondrial respiration.
Traditional measurements of mitochondrial function or glycolysis require an oxygen electrode, or kits and dyes that utilize colorimetric or fluorimetric detection (Li and Graham, 2012; TeSlaa and Teitell, 2014). Most of these methods are invasive and cumbersome methods that only allow low sample throughput. In contrast, the Seahorse analyzer assay with its sensor cartridge system enables measurement of mitochondrial respiration and glycolysis in real time and in a non-invasive manner that does not require any dyes or labels. Cellular energy metabolism research is highly topical in all fields of mammalian cell biology. The following protocol was developed for researchers in cancer biology but with approaches that suit studies of energy metabolism in all mammalian cell systems.
Materials and Reagents
- CELLSTAR® Tissue Culture Plates, 96-well (Greiner Bio One International, catalog number: 655180 )
- Sterile racked pipette tips (1 ml and 200 μl) (VWR, catalog numbers: 613-0738 ; 613-0742 )
- Sterile basins (Corning, Costar®, catalog number: 4870 )
- Sterile reagent tubes (15 and 50 ml) (VWR, catalog numbers: 89039-668 ; 89039-662 )
- Sterile Serological pipettes (5, 10, 25, 50 ml) (Fisher Scientific, catalog numbers: 13-678-11 , 13-678-11D , 13-678-11E , 13-678-11F )
- Glass bottles (500 ml) (Fisher Scientific, catalog number: FB8001000 )
- HCT-116, SK-Mel-5 and SK-Mel-28 cells (ATCC, catalog numbers: CCL-247 , HTB-70 , HTB-72 )
- Seahorse XF24 FluxPak (including sensor cartridges, tissue culture plates, calibrant solution and calibration plates) (Agilent Technologies, Santa Clara, CA)
- Trypsin/EDTA (0.25%/2.21 mM) (Corning, catalog number: 25-053-Cl )
- 1x Ca2+/Mg2+-free DPBS (Thermo Fisher Scientific, GibcoTM, catalog number: 14190250 )
- Liquid Dulbecco’s modified Eagle’s medium (DMEM) (Corning, catalog number: 10-013 )
- Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150 )
- Penicillin/streptomycin solution 100x (Corning, catalog number: 30-002-Cl )
- Powder Dulbecco’s modified Eagle’s medium (DMEM) without Na2HCO3, without HEPES (Corning, catalog number: 50-013 )
- Sodium hydroxide (NaOH) (VWR, catalog number: 97064-476 )
- Oligomycin (Merck, catalog number: 495455-10MG )
- DMSO (VWR, catalog number: BDH1115-1LP )
- FCCP (Cayman Chemical, catalog number: 15218 )
- Rotenone (Cayman Chemical, catalog number: 13995 )
- Antimycin A (Enzo Life Sciences, catalog number: ALX-380-075-M005 )
- Culture media (10% (v/v) FBS) (see Recipes)
- Assay media (see Recipes)
- NaOH (1 M) (see Recipes)
- Oligomycin (10 µM) (see Recipes)
- FCCP (5 µM) (see Recipes)
- Rotenone (5 µM)/antimycin A (5 µM) (see Recipes)
Equipment
- Hemacytometer (Hausser Scientific, catalog number: 1490 )
- Biological Safety Cabinet Class II, Type A2 (NuAire, model: NU-425-400ES )
- Seahorse XF Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA)
- Pipet-Lite Pipette XLS STD 20 XLS (Mettler Toledo, Rainin, model: SL-2XLS+ )
- Pipet-Lite Pipette XLS STD 200 (Mettler Toledo, Rainin, model: SL-200XLS+ )
- Pipet-Lite Pipette XLS 1000 (Mettler Toledo, Rainin, model: SL-1000XLS+ )
- Multichannel Pipet-Lite Pipette XLS 8-CH 1200 (Mettler Toledo, Rainin, model: L8-1200XLS+ )
- Multichannel Pipet-Lite Pipette XLS 8-CH 200 (Mettler Toledo, Rainin, model: L8-200XLS+ )
- Aspirator pump
- Humidified non-CO2 incubator (XF Prep Station; Agilent Technologies, Santa Clara, CA)
- Shallow water bath (Thermo Fisher Scientific, Thermo ScientificTM, model: Precision 180 )
- Pipette controller (BrandTech Scientific, model: Accu-Jet® Pro , catalog number: 26330)
- Humidified, 37 °C, 5% CO2 incubator (Eppendorf, model: Galaxy® 170 R )
- -20 °C biomedical freezer (Sanyo, model: MDF-U731M )
- Autoclave (Consolidated Sterilizer Systems, model: SSR-3A , ADVPB)
- Inverted light microscope (Nikon Instruments, model: Eclipse TS100 )
- pH-meter with semi-micro electrode (Thermo Fisher Scientific, Thermo ScientificTM, model: Orion StarTM A211 , with ROSS 8103BN electrode: (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 8103BN )
Software
- Seahorse Bioscience XF24 software
- Excel (Microsoft)
- GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Plitzko, B. and Loesgen, S. (2018). Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio-protocol 8(10): e2850. DOI: 10.21769/BioProtoc.2850.
- Plitzko, B., Kaweesa, E. N. and Loesgen, S. (2017). The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells. J Biol Chem 292(51): 21102-21116.
分类
癌症生物学 > 细胞能量学 > 细胞生物学试验 > 新陈代谢
细胞生物学 > 细胞新陈代谢 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link