- EN - English
- CN - 中文
Heterologous Expression and Purification of the CRISPR-Cas12a/Cpf1 Protein
CRISPR-Cas12a/Cpf1的异源表达和纯化
发布: 2018年05月05日第8卷第9期 DOI: 10.21769/BioProtoc.2842 浏览次数: 21754
评审: Renate WeizbauerRainer MelzerPeter E Burby
Abstract
This protocol provides step by step instructions (Figure 1) for heterologous expression of Francisella novicida Cas12a (previously known as Cpf1) in Escherichia coli. It additionally includes a protocol for high-purity purification and briefly describes how activity assays can be performed. These protocols can also be used for purification of other Cas12a homologs and the purified proteins can be used for subsequent genome editing experiments. 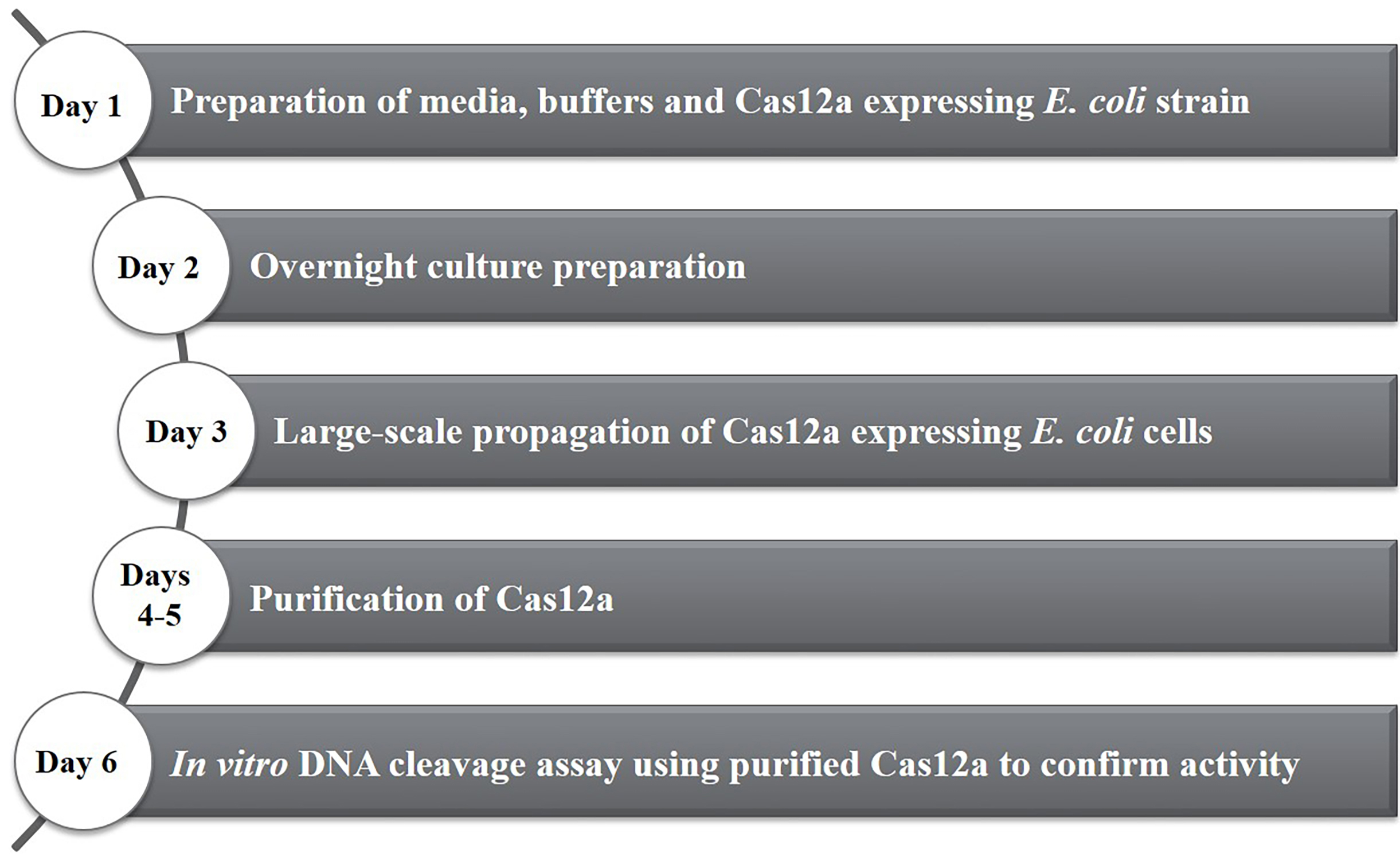
Figure 1. Timeline of activities for the heterologous expression and purification of Francisella novicida Cas12a (FnCas12a) from Escherichia coli
Background
Prokaryotic CRISPR-Cas immune systems provide protection against viruses and plasmids by using CRISPR RNAs (crRNAs) as a guide for sequence-specific targeting of foreign DNA or RNA (van der Oost et al., 2014; Marraffini, 2015). Class 1 CRISPR-Cas systems (comprising types I, III, and IV) typically form multi-subunit protein-crRNA effector complexes, while the class 2 systems (comprising types II, V, and VI) rely on single crRNA-guided effector nucleases for target interference (Mohanraju et al., 2016).
Effector nuclease enzymes from the Class 2 CRISPR-Cas systems have emerged as efficient and precise tools for genome editing and gene expression control (Mali et al., 2013; Doudna and Charpentier, 2014; Hsu et al., 2014). The widely used Cas9, which is the signature protein of type II systems, utilizes a dual guide RNA structure consisting of crRNA and a trans-activating crRNA (tracrRNA) for target recognition (Deltcheva et al., 2011). For genome editing purposes, the dual guide RNA is often replaced by a synthetic fusion of the mature crRNA and tracrRNA, resulting in a long single-molecule guide RNA (sgRNA) in which the individual RNAs are fused by a short linker sequence (Jinek et al., 2012). The sequence of the guide RNA allows binding of complementary DNA targets by base pairing with the target strand, while the other strand of the DNA is displaced. Upon finding a cognate DNA target, the HNH and RuvC nuclease domains of Cas9 mediate cleavage of the target and the displaced strand, respectively (Jinek et al., 2012; Karvelis et al., 2013).
More recently, another novel class 2 CRISPR-Cas nuclease with distinctive features has been identified in bacterial genomes: Cas12a (also known as Cpf1) (Makarova and Koonin, 2015; Zetsche et al., 2015; Shmakov et al., 2017). Cas12a utilizes a single crRNA guide for DNA targeting; it does not require a tracrRNA, resulting in a shorter gRNA sequence compared to the chimeric single-molecule guide RNAs (sgRNA) used by Cas9. While Cas9 requires RNase III-mediated processing of pre-crRNA or individual expression of sgRNAs for the formation of mature guide RNAs, Cas12a can process its own pre-crRNA. This pre-crRNA processing activity allows for simple multiplexing in Cas12a-mediated genome editing (Wang et al., 2017; Zetsche et al., 2017). Whereas Cas9 generates double stranded DNA breaks (DSBs) that are blunt ended, Cas12a generates staggered-end DSBs (Zetsche et al., 2015). Such overhangs can be utilized for overhang-based cloning (Li et al., 2016; Lei et al., 2017). Moreover, Cas9 typically recognizes a G-rich PAM sequence, while all Cas12a orthologues characterized to date recognize a T-rich PAM sequence (Zetsche et al., 2015). Taken together, these features make Cas12a a valuable addition to the genome editing toolbox.
Cas12a has been successfully repurposed for genome editing applications in mammalian cells (Zetsche et al., 2015; Kim et al., 2016a), mice (Hur et al., 2016; Kim et al., 2016b), rice (Endo et al., 2016; Hu et al., 2017; Xu et al., 2017), yeast (Verwaal et al., 2017; Swiat et al., 2017), zebrafish, xenopus (Moreno-Mateos et al., 2017), microalga (Ferenczi et al., 2017) and plant cells (Zaidi et al., 2017; Kim et al., 2017; Tang et al., 2017). The high efficiency and specificity of Cas12a in human cells, coupled with fewer off-target cleavage events compared to Cas9 (Kleinstiver et al., 2016), makes Cas12a a robust and reliable tool for genome editing.
For its in vitro characterization and crystallization (Swarts et al., 2017), Cas12a from Francisella novicida U112 was purified after heterologous expression in Escherichia coli. The expression strain E. coli RosettaTM 2 (DE3) carries a chromosomal T7 RNA polymerase gene under control of an IPTG inducible lacUV5 promoter. The cas12a gene is expressed using a pET vector (Studier and Moffatt, 1986; Rosenberg et al., 1987; Studier et al., 1990) with a lacI-controlled T7 promoter. Here we describe the steps required for controlled expression and purification of FnCas12a. The protocol can also be used for the expression and purification of Cas12a homologs from Acidaminococcus sp. and Lachnospiraceae bacterium.
Materials and Reagents
Note: Equivalent materials and reagents may be used as substitutes.
- Expression of FnCas12a in E. coli RosettaTM 2(DE3)
- 100-ml Erlenmeyer flask (DWK Life Sciences, DURAN®, catalog number: 21 213 24 )
- 2-L Erlenmeyer flask (DWK Life Sciences, DURAN®, catalog number: 21 216 63 )
- 5-L Erlenmeyer flasks (DWK Life Sciences, DURAN®, catalog number: 21 216 73 )
- 50-ml conical centrifuge tubes (Sigma-Aldrich, catalog number: T2318-500EA )
- 2-ml screw top tube (Corning, catalog number: 430659 )
- NalgeneTM PPCO Centrifuge Bottles with Sealing Closure (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3141-0500 ) or equivalent 500-ml centrifuge bottles
- Pipette tips (DeckWorksTM standard pipet tips, Corning, catalog numbers: 4110 ; 4112 ; 4867 )
- 10-ml syringe (BD, catalog number: 309604 )
- 0.22 μm syringe filter (Mdi, catalog number: SYPL0601MNXX204 )
- 250-ml bottle (Greiner Bio One International, catalog number: 227261 )
- Escherichia coli RosettaTM 2(DE3) cells (Merck, Novagen, catalog number: 71400 ) [encodes a T7 RNA polymerase gene under control of a lacUV5 promoter]
- Plasmid pDS015* [pET His6 TEV LIC cloning vector (Addgene, catalog number: 29653 ), with F. novicida U112 cas12a gene insert fused to an N-terminal His-tag; expression under the control of a lacI-controlled T7 promoter]
*Note: Acidaminococcus sp. BV3L6 Cas12a (AsCas12a) and Lachnospiraceae bacterium ND2006 Cas12a (LbCas12a) proteins can also be purified using this protocol with expression vectors 6His-MBP-TEV-huAsCpf1 (Addgene, catalog number: 90095 ) and 6His-MBP-TEV-huLbCpf1 (Addgene, catalog number: 90096 ) - Tryptone (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: LP0042B )
- Yeast extract (BD, BactoTM, catalog number: 212720 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-10 )
- Sodium hydroxide (NaOH) (Merck, EMD Millipore, catalog number: 106462 )
- Ethanol (Fisher Scientific, catalog number: BP2818500 )
- Chloramphenicol (Fisher Scientific, catalog number: BP904100 )
- Kanamycin sulfate (Thermo Fisher Scientific, catalog number: 11815024 )
- Glycerol (Fisher Scientific, catalog number: BP229-4 )
- IPTG (Fisher Scientific, catalog number: BP1755-1 )
- Agar (Acros Organics, catalog number: 400400050 )
- LB medium (see Recipes)
- 1,000x chloramphenicol solution (34 mg/ml) (see Recipes)
- 1,000x kanamycin solution (50 mg/ml) (see Recipes)
- 1 M IPTG (IsoPropyl-1-Thio-β-D-Galactopyranoside) (see Recipes)
- Glycerol stock (50% solution) (see Recipes)
- 100-ml Erlenmeyer flask (DWK Life Sciences, DURAN®, catalog number: 21 213 24 )
- Purification of FnCas12a
- 5 ml HisTrap HP (GE Healthcare, catalog number: 17524701 )
- Dialysis tubing, high retention seamless cellulose tubing, avg. flat width 23 mm (0.9 in.), MWCO 12,400, 99.99% retention (Sigma-Aldrich, catalog number: D0405 )
- Dialysis tubing clamps (Sigma-Aldrich, catalog number: Z371092 )
- 5 ml HiTrap Heparin HP (GE Healthcare, catalog number: 17040601 )
- Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-100 membrane (Merck, EMD Millipore, catalog number: UFC9100 )
- HiLoad 16/600 Superdex 200 pg (GE Healthcare, catalog number: 28989335 )
- GosselinTM Round-Base 10-ml Test Tubes (Corning, GosselinTM, catalog number: TP10-01 ) or other equivalent fraction collection tubes
- NalgeneTM Oak Ridge High-Speed Centrifuge Tubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3114-0050 ) or equivalent 50-ml centrifuge tubes
- Membrane Filter, mixed cellulose esters (Merck, MF-Millipore, catalog number: HAWP04700 )
- Membrane Filter, mixed cellulose esters (Merck, MF-Millipore, catalog number: GSWP04700 )
- Cell pellet from overnight culture in which FnCas12a was expressed (from Procedure A)
- cOmpleteTM, EDTA-free Protease Inhibitor Cocktail (Sigma-Aldrich, Roche Diagnostics, catalog number: 11873580001 )
- Lysozyme from chicken egg white (Sigma-Aldrich, catalog number: L6876-5G )
- β-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- TEV protease (Sigma-Aldrich, catalog number: T4455 )
- 12% Mini-PROTEAN® TGXTM Precast Protein Gels (Bio-Rad Laboratories, catalog number: 4561043 )
- 4x Laemmli protein sample buffer for SDS-PAGE (Bio-Rad Laboratories, catalog number: 1610747 )
- Bio-SafeTM Coomassie Stain (Bio-Rad Laboratories, catalog number: 1610786 )
- PageRuler Prestained Protein Ladder, 10 to 250 kDa (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 26619 )
- Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632 )
- Ethylenedinitrilotetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-10 )
- Tris (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 17926 )
- Imidazole (Sigma-Aldrich, catalog number: I0250 )
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 258148 )
- Potassium chloride (KCl) (Merck, EMD Millipore, catalog number: 104933 )
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 757551 )
- Glycine (Sigma-Aldrich, catalog number: G8898 )
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771 )
- 1 M DTT (Dithiothreitol) stock (see Recipes)
- 0.5 M EDTA (Disodium Ethylene Diamine Tetra-Acetate) stock (pH 8) (see Recipes)
- Lysis Buffer (see Recipes)
- Wash Buffer (see Recipes)
- Elution Buffer (see Recipes)
- Dialysis Buffer (see Recipes)
- Dilution Buffer (see Recipes)
- IEX-A Buffer (see Recipes)
- IEX-B Buffer (see Recipes)
- SEC Buffer (see Recipes)
- 10x SDS-PAGE Electrophoresis Running Buffer (see Recipes)
- 5 ml HisTrap HP (GE Healthcare, catalog number: 17524701 )
- Activity assay using purified Cas12a
- Purified Cas12a Nuclease (from Procedure B)
- Nuclease-free water
- Proteinase K, Molecular Biology Grade (New England Biolabs, catalog number: P8107S )
- crRNA containing the targeting sequence complementary to the target DNA
Note: The RNA can be ordered as a desalted RNA oligonucleotide or as PAGE-purified RNA oligonucleotide from an RNA synthesis company such as Sigma-Aldrich or IDT. - DNA substrate containing the target sequence and a 5’ TTTN PAM sequence
Note: The substrate DNA can be circular or linearized plasmid, PCR products, or synthesized oligonucleotides). As an example, the DNA substrate and crRNA used in the activity assay is shown in Figure 2.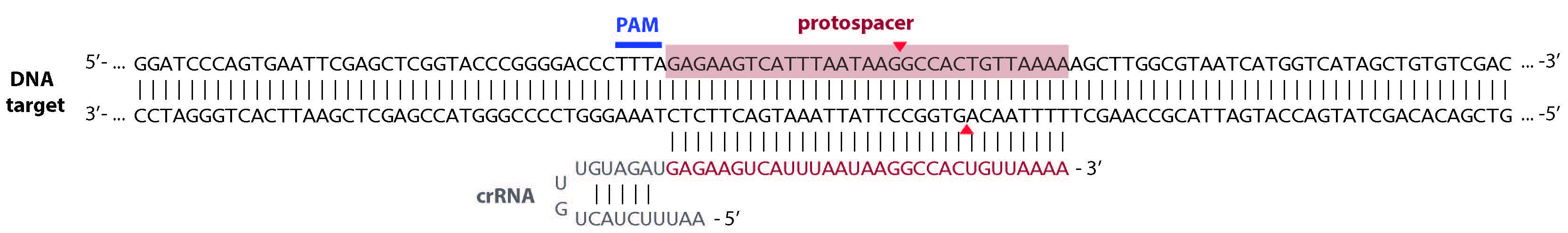
Figure 2. Schematic of the Cas12a crRNA-DNA-targeting complex. The expected cleavage sites are indicated by red arrows. - GeneRuler 1 kb DNA Ladder (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: SM0311 ) or equivalent
- DNA gel Loading Dye [e.g., 6x DNA Loading Dye (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: R0611 )]
- InvitrogenTM SYBRTM Safe DNA Gel Stain (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: S33102 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-10 )
- Magnesium chloride hexahydrate (MgCl2·6H2O)
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- Ethylenedinitrilotetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 258148 )
- 10x Nuclease Reaction Buffer (see Recipes)
- Purified Cas12a Nuclease (from Procedure B)
Equipment
Note: Equivalent equipment can be used.
- Expression of FnCas12a in E. coli RosettaTM 2
- Pipettes (Corning, model: LambdaTM Plus Single-Channel Pipettor, catalog numbers: 4070 ; 4074 ; 4075 )
- New BrunswickTM Innova® 42 incubator (Eppendorf, New BrunswickTM, model: Innova® 42 , catalog number: M1335-0002) or an equivalent incubator that can be set at 37 °C
- Sorvall LYNX 4000 Superspeed Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: Sorvall LYNX 4000 , catalog number: 75006580) or an equivalent centrifuge that can be cooled down to 4 °C and can perform up to 6,000 x g
- New BrunswickTM Innova® 44/44R (Eppendorf, New BrunswickTM, model: Innova® 44/44R , catalog number: M1282-0002) or any equivalent shaker incubator where the temperature can be set at 37 °C and 18 °C
- Cell density meter (GE Healthcare, model: UltrospecTM 10 , catalog number: 80-2116-30), or equivalent spectrophotometer that can measure the density of cells in suspension at 600 nm
- Ice-water bath (water and ice mixed)
- Pipettes (Corning, model: LambdaTM Plus Single-Channel Pipettor, catalog numbers: 4070 ; 4074 ; 4075 )
- Purification of FnCas12a
- SONOPULS HD (Bandelin electronic, model: HD 3200 ) with VS 70 T Sonotrode (Bandelin) or equivalent ultrasonic homogenizer/Sonifier, or alternatively a French Pressure Cell (French Press) for cell lysis
- Peristaltic pump P-1 with connectors for 5 ml HisTrap HP (GE Healthcare, model: Peristaltic Pump P-1, catalog number: 18111091 ) or an equivalent peristaltic pump
- Tubing Connectors for Use with Peristaltic Pump P-1 (GE Healthcare, catalog number: 11300082 )
- ÄKTApurifier 10 FPLC system (GE Healthcare, model: ÄKTApurifier 10 , catalog number: 28406264) or an equivalent FPLC system
- Sorvall LYNX 4000 Superspeed Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: Sorvall LYNX 4000 , catalog number: 75006580) or an equivalent centrifuge that can be cooled down to 4 °C and can perform up to 30,000 x g
- pH meter (QiS, model: B210 )
- Filter holder assembly for filtration (Merck, catalog number: XX1014700 or Nalgene, Thermo Fisher Scientific, Thermo ScientificTM, catalog number: DS0320-2545 ), or equivalent filter holder assembly
- Diaphragm Vacuum Pumps LABOPORT® N 820 (ABM van Zijl B.V, catalog number: ABMK N8203FT18 ), or an equivalent vacuum pump
- Nanodrop (Thermo Fisher Scientific, Thermo ScientificTM, model: NanoDropTM 2000 , catalog number: ND-2000)
- Mini-PROTEAN Tetra cell (Bio-Rad Laboratories, model: Mini-PROTEAN Tetra Cell, catalog number: 1658004EDU ), or an equivalent vertical electrophoresis system
- Epson Perfection V850 Pro scanner (Epson, model: Perfection V850 Pro ) or equivalent scanner or imager suitable for SDS-PAGE gel imaging.
- SONOPULS HD (Bandelin electronic, model: HD 3200 ) with VS 70 T Sonotrode (Bandelin) or equivalent ultrasonic homogenizer/Sonifier, or alternatively a French Pressure Cell (French Press) for cell lysis
- Activity assay using purified Cas12a
- EppendorfTM 5424 Microcentrifuge (Eppendorf, model: 5424 , catalog number: 022620498)
- MUPID One Horizontal Electrophoresis System (Bulldog Bio, catalog number: MU2 ) or an equivalent horizontal electrophoresis system
- G:BOX F3 (Syngene, model: G:BOX F3 , catalog number: 05-GBOX-F3) gel doc system or equivalent DNA agarose gel imaging equipment
- EppendorfTM 5424 Microcentrifuge (Eppendorf, model: 5424 , catalog number: 022620498)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mohanraju, P., Oost, J. V. D., Jinek, M. and Swarts, D. C. (2018). Heterologous Expression and Purification of the CRISPR-Cas12a/Cpf1 Protein. Bio-protocol 8(9): e2842. DOI: 10.21769/BioProtoc.2842.
分类
微生物学 > 异源表达系统 > 大肠杆菌
微生物学 > 微生物生物化学 > 蛋白质 > 活性
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














