- EN - English
- CN - 中文
Cobblestone Area-forming Cell Assay of Mouse Bone Marrow Hematopoietic Stem Cells
小鼠骨髓造血干细胞的鹅卵石样区域形成细胞测定
发布: 2018年05月05日第8卷第9期 DOI: 10.21769/BioProtoc.2824 浏览次数: 10881
评审: Giusy TornilloNandini MondalAnonymous reviewer(s)
Abstract
Bone Marrow Hematopoietic Stem Cells (HSCs) require bone marrow microenvironment for their maintenance and proliferation. Culture of Bone Marrow Mesenchymal Stromal Cells (MSCs) provides appropriate environmental signals for HSCs survival in vitro. Here, we provide a detailed protocol that describes culture conditions for MSCs, flow cytometric isolation of HSCs from mouse bone marrow, and perform co-culture of MSCs and HSCs known as Cobblestone area-forming cell (CAFC) assay. Altogether, CAFC assays can be used as a high-throughput in vitro screening model where efforts are made to understand and develop therapies for complex bone marrow diseases. This protocol needs 3 to 4 weeks starting from culturing MSCs, isolating LSK cells (HSCs), and to performing limited dilution CAFC assay.
Keywords: Mesenchymal Stromal Cells (间充质基质细胞)Background
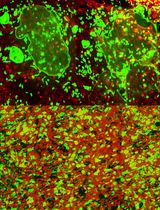
The proliferative, survival and differentiation potential of HSCs is very much dependent on its microenvironment also known as niche. The bone marrow MSCs support the HSCs to keep them in a quiescent state in the bone marrow niche. The intrinsic and extrinsic signals received by the niche contribute to the differentiation of HSCs into mature blood-cell lineages also known as hematopoiesis, without inducing aberrant expansion (Yoshihara et al., 2007; Spindler et al., 2014; Hu et al., 2016). The Cobblestone-Area-Forming Cell Assay (CAFC Assay) is an in vitro co-culture assay of long-term bone marrow HSCs and MSCs. While MSCs are cultured to complete confluence in a tissue culture dish, HSCs are plated over MSCs (de Haan and Ploemacher, 2002). CAFC assays are comparable to in vivo studies of bone marrow and can be used as a rapid screening assay to test the stem cell activity of HSCs and supportive activity of MSCs (Ploemacher et al., 1989). There is a high demand for high throughput screening models that reflect the complex physiology or pathology of the bone marrow microenvironment both in native state or disease models respectively. In this regard, large scale screening was made possible using the HSCs-stroma co-culture system to identify small-molecule inhibitors to develop an effective therapy for acute leukemia (Hartwell et al., 2013). Further, a co-culture system was applied as a model to study several diseases like Fanconi anemia (FA), where it was identified that FA MSCs produce elevated levels of metabolites like glycerophospholipids, which can skew the normal HSCs function (Amarachintha et al., 2015). Further, MSCs provided an impaired environment for HSCs proliferation in patients with aplastic anemia suffering with pancytopenia and in the patients who are in remission after immunosuppression (Schrezenmeier et al., 1996). Further, MSCs from T-cell lymphocytic leukemia mouse model showed adverse proliferation and differentiation capacity of HSCs (Lim et al., 2016). However, in Cord Blood transplants, Cord Blood-MSC co-culture holds promise for successful expansion of cord blood and surge the engraftment in recipients (Denning-Kendall et al., 2003; Robinson et al., 2006). Although several theories were proposed to characterize the isolation and culture of MSCs, ‘The International Society for Cellular Therapy’ has set the minimal criteria for defining ‘multipotent mesenchymal stromal cells’. MSCs derived from bone marrow must be plastic-adherent in standard culture conditions, express cell surface markers, and must differentiate to osteoblasts, adipocytes, and chondroblasts in vitro (Dominici et al., 2006; Keating, 2012). Adhering to these principles, we identified a simplistic approach to culture MSCs from mouse bone marrow and performed limited dilution CAFC assay to enable rapid screenings.
Materials and Reagents
- Pipette tips (USA Scientific, catalog number: 1126-7810 )
- 1,000 µl large orifice pipette tip (USA Scientific, catalog number: 1011-9000 )
- BD Precision glide needles (BD, catalog number: 305155 )
- BD Slip Tip Sterile Syringe 1 ml (BD, catalog number: 309659 )
- BD Single-use Needles 22 G (BD, catalog number: 305159 )
- Falcon 15 ml conical centrifuge tubes (Corning, Falcon®, catalog number: 352097 )
- Falcon standard tissue culture dishes (100 mm culture dish) (Corning, Falcon®, catalog number: 353003 )
- Falcon 35 mm TC-treated Easy-Grip style cell culture dish (Corning, Falcon®, catalog number: 353001 )
- Nunc Edge 2.0 96-well cell culture plates (Thermo Fischer Scientific, Thermo ScientificTM, catalog number: 167425 )
- 3-well chamber slide (IBIDI, catalog number: 80381 )
- Falcon Polystyrene Microplates 6-well plate TC-treated (Corning, Falcon®, catalog number: 353934 )
- C57BL/6J mice of age 4 to 8 weeks old (THE JACKSON LABORATORY, catalog number: 000664 )
- 70% ethanol
- Red blood cell lysis buffer (Sigma-Aldrich, Roche Diagnostics, catalog number: 11814389001 )
- Trypsin-EDTA (0.25%), phenol red (Thermo Fischer Scientific, GibcoTM, catalog number: 25200056 )
- Mouse MSC Functional Identification Kit containing antibodies Osteopontin, Fabp4, and Collagen II (R&D Systems, catalog number: SC010 )
- DPBS (10x), no calcium, no magnesium (Thermo Fischer Scientific, GibcoTM, catalog number: 14200075 )
- 16% formaldehyde (w/v), methanol-free (Thermo Fischer Scientific, Thermo ScientificTM, catalog number: 28906 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418 )
- 4’,6-Diamidino-2-phenylindole (DAPI) (Thermo Fischer Scientific, InvitrogenTM, catalog number: D1306 )
- VECTASHIELD Antifade mounting medium (Vector Laboratories, catalog number: H-1000 )
- Ficoll-Paque PLUS (GE Healthcare, catalog number: 17144002 )
- Streptavidin APC-Cy-7 (BD, BD Biosciences, catalog number: 554063 )
- PE rat anti-mouse Ly-6A/E (BD, BD Biosciences, catalog number: 561076 )
- Rat anti-mouse CD117 (BD, BD Biosciences, catalog number: 561074 )
- V450 mouse lineage antibody cocktail (BD, BD Biosciences, catalog number: 561301 )
- Fetal bovine serum, qualified, heat inactivated, USDA-approved (FBS) (Thermo Fischer Scientific, GibcoTM, catalog number: 10438034 )
- Iscove’s modified Dulbecco’s medium (Thermo Fischer Scientific, InvitrogenTM, catalog number: 12440053 )
- Bovine calf serum (GE Healthcare, Hyclone, catalog number: SH30072.03 )
- Epidermal growth factor (R&D Systems, catalog number: 2028-EG-200 )
- Platelet-Derived growth factor (R&D Systems, catalog number: 220-BB-010 )
- Penicillin-streptomycin (Thermo Fischer Scientific, GibcoTM, catalog number: 15140122 )
- 2-Mercaptoethanol (Thermo Fischer Scientific, GibcoTM, catalog number: 21985023 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
- Donkey serum (Sigma-Aldrich, catalog number: D9663 )
- Bone marrow wash buffer (see Recipes)
- MSCs media (see Recipes)
- DPBS blocking solution (see Recipes)
Equipment
- ErgoOne 100-1,000 µl Single Channel Pipettes (USA Scientific, model: 7110-1000 )
- Eppendorf 5804 Benchtop Centrifuge (Eppendorf, model: 5804 )
- BD FACSCanto II to analyze the hematopoietic stem cells (BD, model: BD FACSCanto II )
- BD FACSAria II to sort the hematopoietic stem cells (BD, model: BD FACSAria II )
- NIKON Eclipse 90i microscope to capture immunofluorescence images (Nikon Instruments, model: Eclipse 90i )
- OLYMPUS IX53 Inverted Microscope to capture phase contrast images of cobblestone area (Olympus, model: IX53 )
Software
- BD FACSDiva v8.0.1 Software (BD Biosciences)
- FlowJo software (FLOW JO)
- CellSens software (Olympus)
- GraphPad Prism software
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Amarachintha, S. and Pang, Q. (2018). Cobblestone Area-forming Cell Assay of Mouse Bone Marrow Hematopoietic Stem Cells. Bio-protocol 8(9): e2824. DOI: 10.21769/BioProtoc.2824.
分类
干细胞 > 成体干细胞 > 造血干细胞
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link