- EN - English
- CN - 中文
Sebinger Culture: A System Optimized for Morphological Maturation and Imaging of Cultured Mouse Metanephric Primordia
Sebinger培养:一个用于小鼠后肾原基形态成熟和成像的优化系统
发布: 2018年02月20日第8卷第4期 DOI: 10.21769/BioProtoc.2730 浏览次数: 7326
评审: Alessandro DidonnaXia WangAnonymous reviewer(s)

相关实验方案
用于研究肺部感染与免疫反应的离体肺组织培养模型,以 SARS-CoV-2 为 RNA 病毒研究示例
Elena V. Maryukhnich [...] Elena J. Vasilieva
2025年12月20日 709 阅读
Abstract
Here, we present a detailed protocol on setting up embryonic renal organ cultures using a culture method that we have optimised for anatomical maturation and imaging. Our culture method places kidney rudiments on glass in a thin film of medium, which results in very flat cultures with all tubules in the same image plane. For reasons not yet understood, this technique results in improved renal maturation compared to traditional techniques. Typically, this protocol will result in an organ formed with distinct cortical and medullary regions as well as elongated, correctly positioned loops of Henle. This article describes our method and provides detailed advice. We have published qualitative and quantitative evaluations on the performance of the technique in Sebinger et al. (2010) and Chang and Davies (2012).
Keywords: Organ culture (器官培养)Background
The metanephric (permanent) kidneys of mammals develop from simple rudiments located at the caudal end of the intermediate mesoderm. In mice, at about embryonic day (‘E’) 10 these rudiments form and consist of two morphologically distinguishable components; an epithelial ureteric bud that arises as a diverticulum of the Wolffian (nephric) duct, and a metanephrogenic mesenchyme that forms next to the duct. As development progresses, the ureteric bud enters the metanephrogenic mesenchyme and undergoes many successive rounds of growth and branching to make a ‘tree’; this later remodels to produce a mature collecting duct system in which tubules radiate from a central cavity, the renal pelvis (Lindstrom et al., 2015). The renal pelvis drains to the ureter, which forms from the original stalk of the ureteric bud. As the ureteric bud develops, it induces cells from the metanephrogenic mesenchyme to condense around each of its tips to form a ‘cap mesenchyme’ (Schreiner, 1902; Reinhoff, 1922). The cap mesenchymes are stem cell populations that divide as the tips divide, so that each tip formed by bifurcation of an existing branch inherits its own cap (reviewed by Hendry et al., 2011). Cells at the more distal ends of the caps differentiate to form excretory nephrons, which connect to the ureteric bud branch from whose cap they formed (Georgas et al., 2009). Blood vessels invade from the base of the metanephros and follow the ureteric bud, making a network around (but never entering) the cap mesenchymes (Munro et al., 2017): later, these vessels will serve glomeruli and other parts of the kidney.
In vitro culture of metanephric kidney rudiments has a long history. Indeed, these were among the first embryonic organs to show continued development outside the body (Carrel and Burrows, 1910). The continued development of kidney rudiments outside of the body was a scientific, as well as a technical, advance: the autonomous development of isolated organs demonstrated that the information required to build them was ‘local’ and did not depend on the rest of the embryo. This observation added considerable support to the idea that architecture of the body is hierarchical, with modules (organs) that largely look after themselves and interact with the rest of the body only at specific functional interfaces. The earliest culture methods used rather complex media and culture supports, such as Grobstein’s use of clotted avian plasma and chick embryo extract (Grobstein, 1953). These systems were necessary because simply placing a kidney rudiment in a glass dish or flask resulted in its breakdown because cells adhered to the substrate and spread out to form a monolayer. Immersion in medium in non-adhesive dishes prevents cell dispersal but does not result in proper development (see ‘Data analysis’ section). Both imaging and reproducibility were greatly improved by Saxén’s adoption of a culture method developed by Trowell for culture of rat lymph nodes (Trowell, 1954). Saxén placed embryonic kidney rudiments, isolated from mice at E11, on filters that were supported by a stainless steel grid at the interface between gas and medium (Saxén et al., 1962).
The Trowell method has been a mainstay of research in kidney development for many decades. It allows for significant ureteric bud growth and branching, formation of nephrons, differentiation of their separate proximal and distal domains and connection of nephrons to ureteric bud branches. Rudiments grow flat enough to facilitate confocal microscopy of fixed specimens without the need for ‘clearing’ techniques, and even conventional epifluorescence microscopy is adequate for many purposes (e.g., Davies et al., 2014). Another advantage is that diffusion paths have proved to be short enough and open enough to enable mechanisms of development to be investigated in this method with drugs (e.g., Fisher et al., 2001), growth factors (e.g., Piscione et al., 1997), function-blocking antibodies (e.g., Falk et al., 1996) and, to a limited extent, siRNAs (e.g., Davies et al., 2004). The Trowell system, together with variants that place rudiments at the air-medium interface using use Transwell filters instead of stainless steel supports, remains very common in studies of kidney development.
Useful as it is, the Trowell culture system has a few problems. One is that the filter itself interferes with bright-field/phase contrast imaging because the filter pores appear, out of focus, in the image (though some Transwell systems do achieve good optical clarity). Another is that the tissue is too thick for reliable high-resolution, unattended time-lapse photography, because tubules leave the focal plane. A third problem is that some aspects of renal development, such as the formation of distinct cortex and medulla, and extension of nephrons’ loops of Henle from the cortex to the medulla, occur poorly if at all. In an attempt to address these issues, we developed an alternative culture system that uses the surface tension of very shallow medium to hold a kidney rudiment on to a clear glass substrate. To our surprise, the system not only solved the imaging problem, but it also allowed the organ rudiments to develop clear cortico-medullary zonation and nephron maturation proceeded as far as the production of clear and elongated loops of Henle (Sebinger et al., 2010) (Figure 1). The enhanced development is seen in cultures made from natural kidneys isolated from mouse embryos, and also in organoids engineered from suspensions of stem cells (Chang and Davies, 2012). 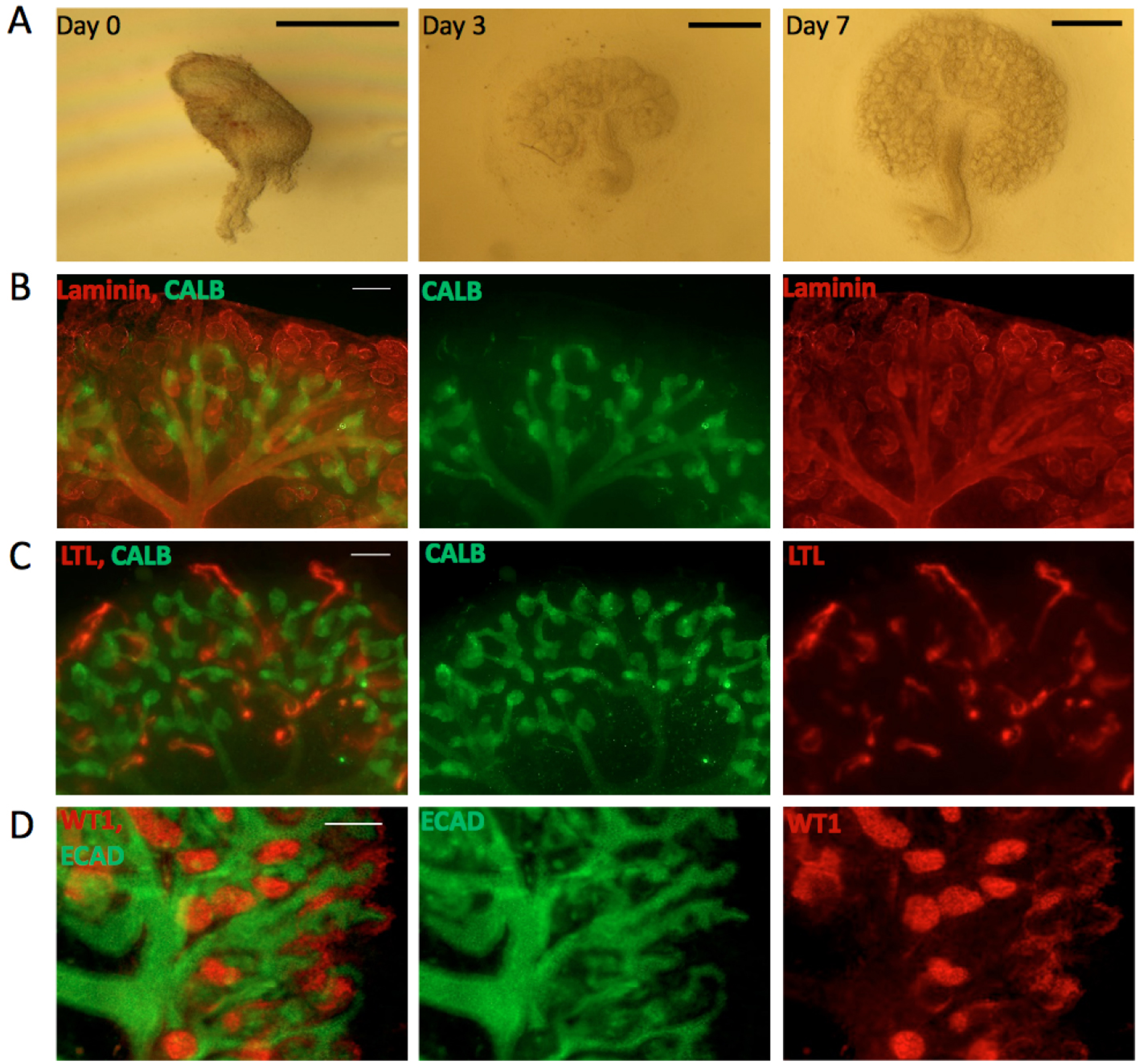
Figure 1. Kidneys cultured in the Sebinger system. A. Bright field images for E11.5 kidney grown in culture for 0, 3 and 7 days. Scale bars = 0.5 mm. B-D. E11.5 kidneys cultured for 7 days in the Sebinger system and stained for different renal markers to show maturation. B. Stained for the ureteric bud marker CALB (shown in green) and the basement membrane marker Laminin (shown in red). The red channel shows the presence of loops of Henle dipping into the medulla; C. Stained for CALB (green) and the proximal tubular marker LTL (red); D. Stained for ECAD (ureteric bud and distal tubular marker; shown in green) and WT1 (podocyte and cap mesenchyme marker; shown in red). Scale bars = 100 μm.
Materials and Reagents
- 1 ml disposable syringes (Plastipak 1 ml, BD, catalog number: 303172 ) with fine needles (0.5 x 16 mm/25 G x 5/8”, Microlance 3, BD, catalog number: 300600 )
- 40 x 0.13 mm borosilicate glass coverslips (VWR, catalog number: 631-0177 )
- Silicone cones (flexiPERM Cone shape A, Greiner Bio One International)
These are at the time of writing available only on special order–phone Greiner–and delivery times can be long enough to make forward planning important. The cones can be re-used for years.
Note: We know of no suitable substitutes. - 100 mm sterile Petri dishes (for dissection–surface quality is irrelevant) (Cell Star®, Greiner Bio One International, catalog number: 664160 )
- 60 x 15 mm sterile Petri dishes (Cell Star®, Greiner Bio One International, catalog number: 628160 )
Note: We use Greiner but expect that others will also be suitable. - Glass Pasteur pipettes (150 mm, Volac, catalog number: D810 )
- Timed-mated mice at E11.5 (E10.5 is also suitable but is a more challenging dissection)
- Sterile distilled water
- 100% methanol
- Eagle’s minimal essential medium with Earle’s salts and non-essential amino acids (Sigma-Aldrich, catalog number: M5650 )
- Foetal bovine serum (FBS) (Biochrom, catalog number: S 0415 )
- Penicillin-streptomycin (Sigma-Aldrich, catalog number: P4333 )
- Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: 79382 )
- Anti-laminin (Sigma-Aldrich, catalog number: L9393 )
- FITC anti-rabbit (Sigma-Aldrich, catalog number: F0382 )
- EmbryoMax® Penicillin-streptomycin solution, 100x (Sigma-Aldrich, catalog number: TMS-AB2-C )
- Hydrogen peroxide (Sigma-Aldrich, catalog number: H1009 )
- Ammonium hydroxide (Sigma-Aldrich, catalog number: A6899 )
- Cleaning solution (see Recipes)
- Dissecting medium (see Recipes)
- Culture medium (see Recipes)
- Hydration buffer (see Recipes)
Equipment
- Forceps
- Scalpel (curved blade, e.g., D-form, type 22, Swann Morton, catalog number: 0108 )
- 80 °C oven
Note: We use a Gallenkamp Hotbox size 1, but any 80 °C oven should be suitable. - Cell culture incubator at 37 °C, 5% CO2
Note: We use a NuAire 5500E (NuAire, model: NU-5500E ), but any stable incubator should work as well. - Dissecting microscopes
Note: We use ZEISS Stemi 2000C microscopes (ZEISS, model: Stemi 2000-C ) with transilluminating stages, but have demonstrated the technique in other laboratories with other models of dissecting microscope. The precise type of microscope is not important, but transillumination (rather than epi-illumination) is. - Clean area
Note: We do not use safety cabinets (for the non-pathogen-infected samples we use), because the vibration of the fans is a nuisance. We do use simple cabinets about the size of a safety cabinet, made from Perspex, to provide shelter from dust moved by Edinburgh winds blowing through ill-fitting antique lab windows).
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Elhendawi, M. and Davies, J. A. (2018). Sebinger Culture: A System Optimized for Morphological Maturation and Imaging of Cultured Mouse Metanephric Primordia. Bio-protocol 8(4): e2730. DOI: 10.21769/BioProtoc.2730.
分类
细胞生物学 > 细胞分离和培养 > 器官培养
发育生物学 > 形态建成 > 器官形成
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link