- EN - English
- CN - 中文
Measuring Mitochondrial ROS in Mammalian Cells with a Genetically Encoded Protein Sensor
用基因编码的蛋白质传感器测定哺乳动物细胞线粒体中的ROS
发布: 2018年01月20日第8卷第2期 DOI: 10.21769/BioProtoc.2705 浏览次数: 10697
评审: Nicoletta CordaniAlexandros AlexandratosBegona Diaz
Abstract
Reactive oxygen species (ROS) are not only known for their toxic effects on cells, but they also play an important role as second messengers. As such, they control a variety of cellular functions such as proliferation, metabolism, differentiation and apoptosis. Thus, ROS are involved in the regulation of multiple physiological and pathophysiological processes. It is now apparent that there are transient and local changes in ROS in the cell; in so-called ‘microdomains’ or in specific cellular compartments, which affect signaling events. These ROS hotspots need to be studied in more depth to understand their function and regulation. Therefore, it is necessary to identify and quantify redox signals in single cells with high spatial and temporal resolution. Genetically encoded fluorescence-based protein sensors provide such necessary tools to examine redox-signaling processes. A big advantage of these sensors is the possibility to target them specifically. Mitochondria are essential for energy metabolism and are one of the major sources of ROS in mammalian cells. Therefore, the evaluation of redox potential and ROS production in these organelles is of great interest. Herein, we provide a protocol for the real-time visualization of mitochondrial hydrogen peroxide (H2O2) using the H2O2-specific ratiometric sensor mitoHyPer in adherent mammalian cells.
Keywords: Mitochondrial ROS (线粒体ROS)Background
ROS are produced as by-products of mitochondrial respiration, through the leakage of electrons from the electron transfer chain. These ROS are considered toxic and cause the oxidation of lipids, proteins, and lead to mitochondrial DNA damage (Ralph et al., 2010; Bogeski and Niemeyer, 2014; Cierlitza et al., 2015; Gibhardt et al., 2016). While mitochondria serve as a hub of metabolism, bioenergetics, and cell death, the emerging role of mitochondrial ROS as second messengers in regulating other cellular functions is also increasingly accepted (Chandel, 2015; Reczek and Chandel, 2015; Shadel and Horvath, 2015; Wilems et al., 2015). To monitor mitochondrial ROS with high spatial and temporal resolution remains challenging due to the short half-life of ROS and the limitation of available probes (Kuznetsov et al., 2011; Norcross et al., 2017). The primary reactive species of mitochondrial origin are superoxide anion, hydroxyl radical, singlet oxygen, and hydrogen peroxide (Gibhardt et al., 2016; Idelchik et al., 2017). Hydrogen peroxide (H2O2) is one of the most stable ROS and is thus an attractive tracking tool for examining the cellular redox state.
During the past decade, several groups designed genetically encoded protein sensors to specifically detect H2O2 (Belousov et al., 2006; Gutscher et al., 2009). The specificity, reversibility, and sensitivity of these protein sensors make them suitable for real-time visualization of H2O2 under a broad range of physiological conditions and stimulations.
The HyPer and roGFP2-Orp1 sensors are advantageous in particular and can be used in various cell systems (Ermakova et al., 2014; Hernandez-Barrera et al., 2013; Bogeski et al., 2016). The HyPer sensor is a combination of a circular permutated yellow fluorescent protein (cpYFP), which is inserted in the regulatory domain of the bacterial H2O2 sensing protein OxyR. The oxidation of cysteine199 found on OxyR initiates conformational changes in HyPer. In a reduced state HyPer has two excitation peaks at 420 nm and 500 nm, and one emission peak at 516 nm. Following oxidation, the peak at 420 nm decreases and the peak at 500 nm increases, thus allowing ratiometric measurement of H2O2. (Bilan and Belousov, 2017). Given that pH fluctuations can also affect the signal from HyPer probes, a mutation at cysteine 199 was introduced to generate a probe named SypHer for monitoring pH, which has the same pH sensitivity but does not react to oxidation (Matlashov et al., 2015; Poburko et al., 2011). The roGFP probe is based on an engineered GFP containing two cysteine residues capable of forming a disulfide bond (Morgan et al., 2011). It has two excitation maxima at 400 and 490 nm with the emission around 510 nm; the ratio of these two excitation maxima depends on the state of the disulfide bond. The development of roGFP probes now provides important alternative tools aimed at detecting H2O2 or the potential of the glutathione redox pair (Gutscher et al., 2008; Kasozi et al., 2013; Habich and Riemer, 2017; Lismont et al., 2017; Müller et al., 2017).
Here we describe a detailed protocol for the real-time imaging and monitoring of mitochondrial H2O2 with the mitoHyPer sensor. The approach can be performed on different cellular systems with a basic understanding of real-time imaging and fluorescence microscopy; the data analysis procedure depends on the software available.
Materials and Reagents
- Round glass coverslips 25 mm No. 1.5 (Kindler/ORSA tec®, Round cover glasses)
- 6-well plates (Corning, Costar®, catalog number: 3516 )
- Falcon tubes (15 ml) (VWR, Corning, catalog number: 62406-200 )
- Serological pipettes (Corning, Costar®, catalog number: 4488 )
- Plasmids
mitoHyPer (Evrogen, catalog number: FP942 )
mitoSypHer (Addgene, catalog number: 48251 ) - Cell growth medium (specific to the cells used in the experiment)
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270106 )
- Fugene® HD (Promega, catalog number: E2312 )
- Opti-MEMTM (Thermo Fisher Scientific, GibcoTM, catalog number: 51985-026 )
- Baysilone paste (VWR, GE Bayer Silicines, catalog number: 291-1210 )
- Accutase (Sigma-Aldrich, catalog number: A6964 ) or Trypsin (Thermo Fisher Scientific, GibcoTM, catalog number: 25300062 )
- 1x DPBS, no calcium, no magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-094 )
- 1,4-Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632 )
- Hydrogen peroxide solution 30% (w/w) in H2O, contains stabilizer (Sigma-Aldrich, catalog number: H1009 )
- Stimulants and inhibitors (these are experiment-dependent)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Potassium chloride (KCl) (VWR, AnalaR NORMAPUR®, catalog number: 26764.298 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Merck, catalog number: 102382 )
- Magnesium chloride (MgCl2) (Merck, catalog number: 105833025 )
- D(+)-Glucose anhydrous (Merck, catalog number: 108337 )
- EGTA (Sigma-Aldrich, catalog number: E4378 )
- 1 M HEPES (Sigma-Aldrich, catalog number: H7523 )
- Ringer buffer (0.25 mM Ca2+, pH 7.4) (see Recipes)
Equipment
- ZTM Series COULTER COUNTER® Cell (Beckman Coulter, model: 6605699 ) and Particle Counter Z1 (Beckman Coulter or any other counting device)
- Incubator with humidity and gas control for cell culture
- Zeiss Axio Observer.Z1 (Carl Zeiss, model: Axio Observer.Z1 ) setup (Figure 1) (Incubation System S includes Temp Module S, CO2 Module S, O2 Module S, Heating Module S)
- Tweezers (e.g., style Dumont Nr. 7)
- Imaging chamber and ring insert (self-made) and perfusion system (Figure 2)
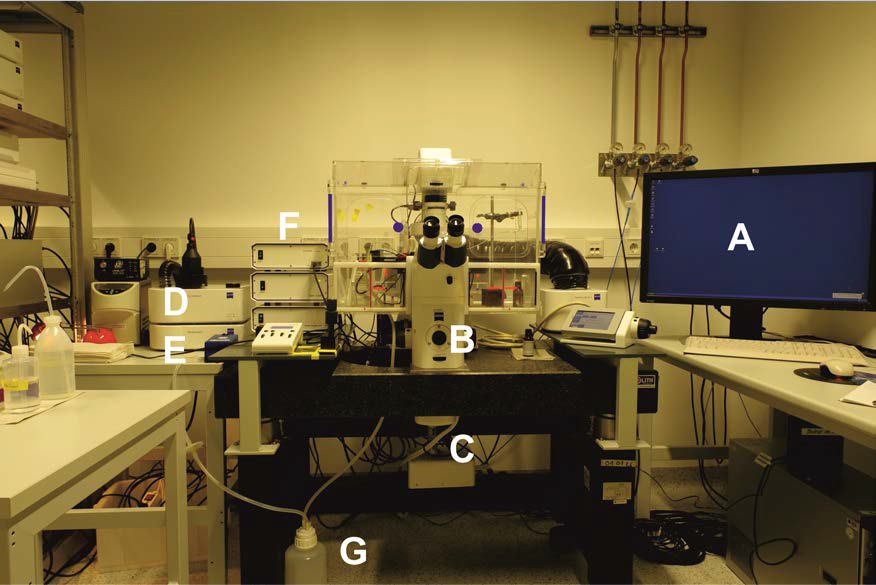
Figure 1. Zeiss Cell Observer.Z1 setup with temperature, CO2 controlling unit, gas chamber and perfusion system. A. Analysis computer; B. Cell Observer.Z1 with 40x oil objective and corresponding filter sets; C. Evolve 512 x 512 EM-CCD camera; D. CO2 supply unit; E. Pecon XL S1 incubator and control modules; F. LED Colibri with corresponding modules. G. Pump and perfusion system.
Notes:- For HyPer measurements, the CFP/YFP filters are essential, but a multiband filter cube with the same property is also a functional option.
- For HyPer experiments, we used the LED light source with the wavelength at 505 nm and 420 nm and corresponding beam splitters.
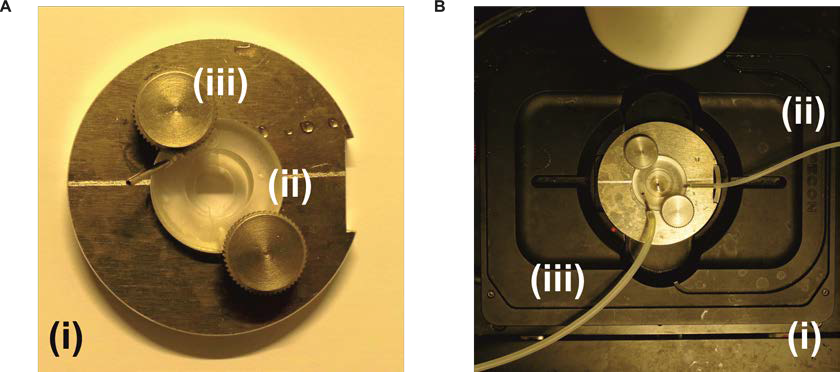
Figure 2. Imaging chamber and mount module with temperature control. A. The self-made imaging chamber (i) with a perfusion chamber plastic insert (ii) that is fixed with knobs (iii). The coverslip with the cells is attached to the lower part of the plastic insert and a small 12 mm coverslip is attached to the upper part of the plastic insert in order to create a small perfusion channel for the measurement. B. The imaging chamber attached to the perfusion system and to the stage of the microscope (i). The perfusion tube (ii) is attached to a syringe in order to add the solutions during the measurement, while the second perfusion tube (iii) is attached to a suction pump system to remove the waste liquid.
- For HyPer measurements, the CFP/YFP filters are essential, but a multiband filter cube with the same property is also a functional option.
Software
- Axiovision 4.6v (Zeiss) with a license for fast acquisition function and measurement analysis or similar
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Zhang, X., Gibhardt, C. S., Cappello, S., Zimmermann, K. M., Vultur, A. and Bogeski, I. (2018). Measuring Mitochondrial ROS in Mammalian Cells with a Genetically Encoded Protein Sensor. Bio-protocol 8(2): e2705. DOI: 10.21769/BioProtoc.2705.
分类
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link