- EN - English
- CN - 中文
Generation of Chemically Induced Liver Progenitors (CLiPs) from Rat Adult Hepatocytes
利用大鼠成年肝细胞生成化学诱导肝祖细胞(CLiP)
发布: 2018年01月20日第8卷第2期 DOI: 10.21769/BioProtoc.2689 浏览次数: 11603
评审: Nicoletta CordaniSara JohnsonXiaoyi Zheng
Abstract
Primary mature hepatocytes (MHs) or their progenitor cells are candidate cell sources for cell transplantation therapy in severe liver diseases. However, stable culture of these cells or generation of equivalent cells from pluripotent stem cells has been limited. Using a cocktail of small molecules that we previously found useful in stable culture of multiple types of stem/progenitor cells, we recently established a novel method to generate bipotent liver progenitor cells, named chemically induced liver progenitors (CLiPs), from adult rat MHs. Here, we describe a detailed protocol for the induction of rat CLiPs. We first describe the method to isolate primary rat MHs and then describe how to induce CLiPs from these MHs. In addition, we describe a method to evaluate the bipotentiality of generated CLiPs to differentiate into hepatocytes and biliary epithelial cells. We also describe how to establish stable CLiPs through long-term culture with detailed example data. Primary CLiPs can be generated within 2 weeks, and stable CLiPs, which undergo 10 passages, can be established within 2.5-4 months with batch-to-batch variability.
Keywords: Hepatocyte (肝细胞)Background
There is a strong demand for a novel cell source to realize regenerative medicine for liver diseases. The only current treatment for end-stage liver diseases is liver transplantation, but its application is limited due to donor shortages. Recently, our group proposed an approach to generate a novel type of LPC that can stably expand in vitro and can repopulate injured liver of chronic hepatitis animal models with an extensive efficiency (Katsuda et al., 2017). Using a cocktail of three small molecules Y-27632, A-83-01 and CHIR99021 (YAC), this approach allows reprogramming of terminally committed MHs into bipotent LPCs without any genetic modification. Thus, we name these cells chemically induced liver progenitors (CLiPs). Here, we describe a step-by-step protocol to generate CLiPs from primary rat MHs and evaluate the bipotentiality of the generated CLiPs (Figure 1).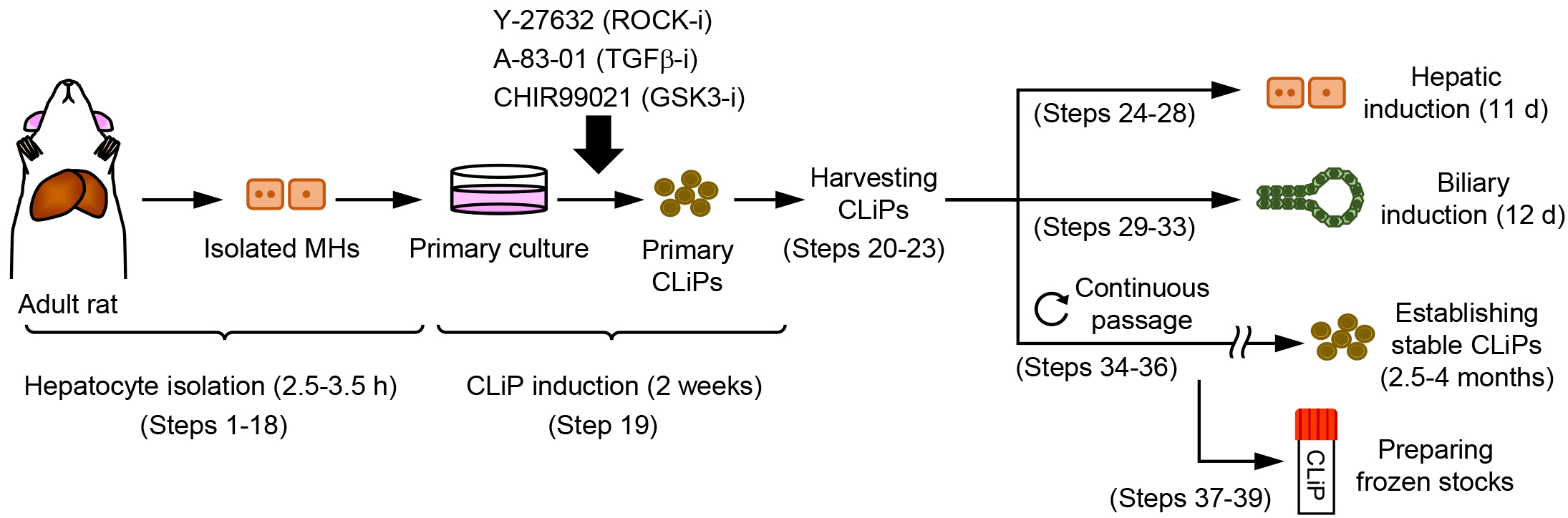
Figure 1. Overview of the experimental workflow. Protocols described in this article are composed of hepatocyte isolation (Steps 1-18), CLiP induction from the primary cultured hepatocytes (Step 19), harvesting the generated primary CLiPs (Steps 20-23), hepatic induction of CLiPs (Steps 24-28), biliary induction of CLiPs (Steps 29-33), establishment of stable CLiPs through continuous passage (Steps 34-36), and freeze stocking of CLiPs (Steps 37-39).
Experimental design and expected results:
Isolation of rat primary MHs (Steps 1-18): Rat primary MHs are isolated using the 2-step collagenase perfusion method (Seglen, 1976). In the first step, blood is eliminated from the liver by perfusing the liver with Hanks’ balanced salt solution (HBSS) supplemented with EDTA and EGTA. In the second step, the liver is digested by perfusing HBSS supplemented with collagenase. Then, the liver is extracted, minced, and further digested ex vivo with the remaining collagenase solution. MHs are collected by low-speed centrifugation at 50-60 x g (our centrifugation machine [Kubota 2800] uses 57 x g with the rotation speed at 600 rpm; researchers should adjust their rotation speed such that they can obtain 50-60 x g centrifugal force). This sequential low-speed centrifugation allows exclusion of nonparenchymal cells, such as biliary epithelial cells, sinusoidal endothelial cells, Kupffer cells and stellate cells, which weigh less than MHs, thereby enabling MH enrichment.
Induction of primary CLiPs using the small molecule cocktail YAC (Step 19): Rat CLiPs can be induced from primary MHs upon stimulation with a combination of three small molecules, YAC. An evident morphological change compared with YAC-free (YAC(-)) culture occurs approximately 4-5 days after plating (Figure 2). As previously reported, rodent MHs can divide once in vitro at approximately 2-4 days even without YAC; their proliferation completely stops thereafter (Loyer et al., 1996; Frémin et al., 2009). In contrast, MHs can continuously proliferate to produce CLiPs during 2-week culture (Figure 2). Although the emergence of CLiPs is highly reproducible (100% in our lab, n > 50), variability in the expandability of CLiPs is noted (Figures 3A and 3B). This variability is not dependent on the viability of primary MHs to be used for reprogramming (Figure 3C). In addition, when we use animals between 9 and 20 weeks of age, we do not observe a correlation between age and the expandability of CLiPs (Figure 3D). In our primary study, to avoid possible contamination of LPCs, which might reside in young animal livers, we used relatively old animals at the age of 10 to 20 weeks (Katsuda et al., 2017). However, when younger animals are used, such as animals at 4 to 8 weeks of age, increased expandability of CLiPs might be obtained.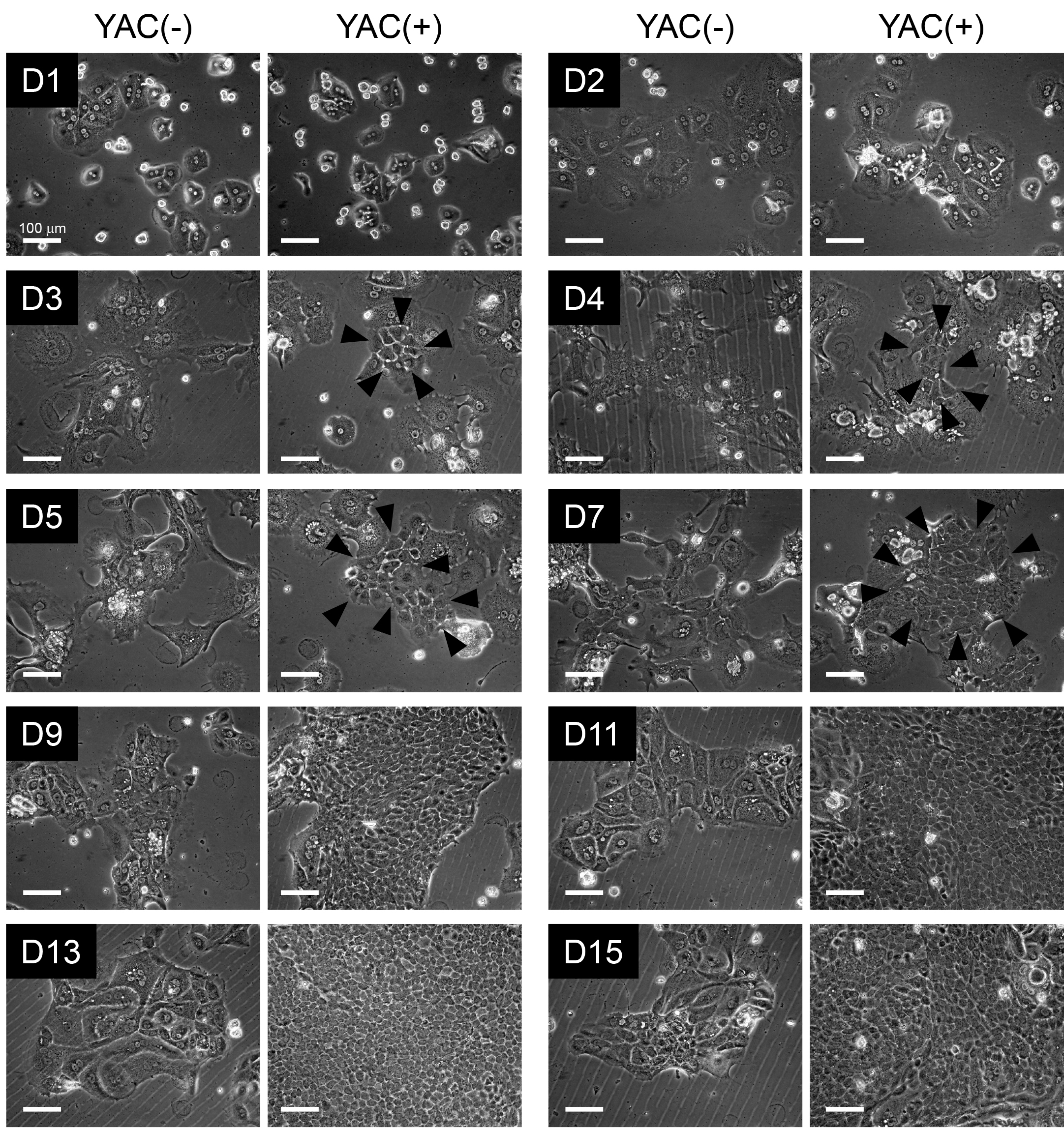
Figure 2. Morphological change of rat MHs during reprogramming to CLiPs. Phase contrast images of rat MHs during 2-week culture in the absence or presence of YAC. On D3, smaller cells (indicated by arrowheads) appear, and the proliferation of these small cells, namely, CLiPs, becomes evident on D4-D5. On approximately D7, CLiP colonies become noticeable, and these colonies become larger thereafter. CLiPs are distinguishable from MHs because their nucleus/cytoplasm ratio is apparently larger than that of MHs. In addition, the size of CLiPs is much smaller than that of MHs.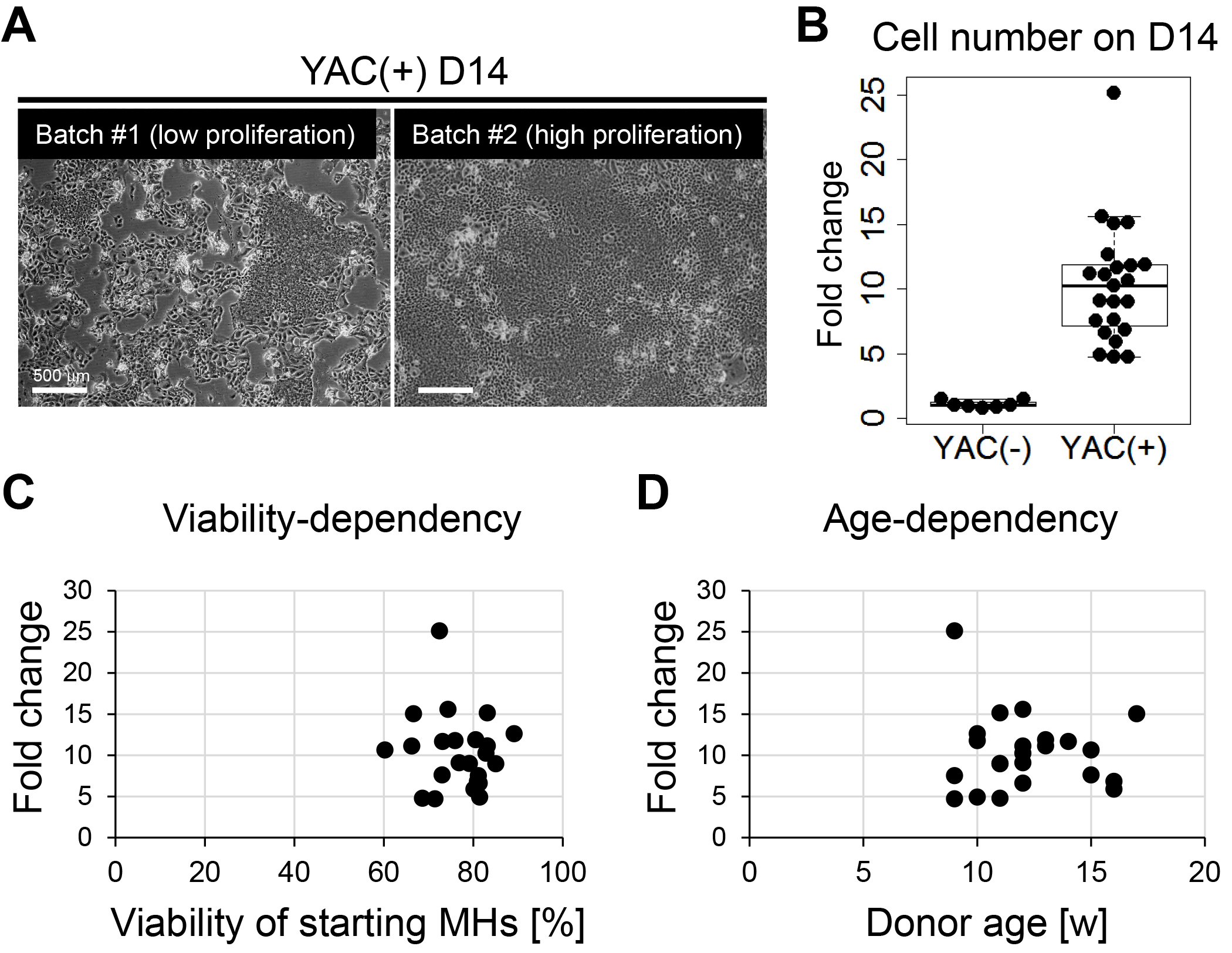
Figure 3. Inter-batch variation in proliferative capacity of primary rat CLiPs. A. Representative image of primary CLiPs on D14 with low expansion capacity (batch #1, left) and those with high expansion capacity (batch #2, right). Although reprogrammed CLiPs can be obtained in both experimental batches, the cell density on D14 is clearly different between these two batches. B. A box-and-whisker plot of cell number on D14 indicates inter-batch variation in proliferative capacity of primary CLiPs (right). The cell number was normalized with the inoculated cell number and is presented as fold change. The fold change of YAC(-) cells is presented as the control (left). n = 7 and 23 for YAC(-) and YAC(+) cells, respectively. C. No correlation was noted between the expansion capacity of primary CLiPs and the viability of inoculated MHs. D. No correlation was noted between the expansion capacity of primary CLiPs and the age (9 weeks to 17 weeks) of donor animals.
Evaluation of bipotentiality of CLiPs (Steps 25-34): Primary CLiPs can be differentiated into both hepatocytes and BECs. Hepatic differentiation is performed based on a previously reported protocol that induced hepatic maturation of foetal liver cells (Kamiya et al., 2002). This protocol consists of two steps. In the first step, CLiPs are cultured for 6 days in SHM + YAC supplemented with 20 ng/ml of oncostatin M and high concentration of dexamethasone (10-6 M). In the second step, CLiPs are further cultured for another 2 days in the presence of Matrigel. Morphological changes in Step 1 are relatively mild. The nucleus/cytoplasm ratio of hepatic-induced cells (Hep-i(+) cells) is reduced, and the cytoplasm becomes rich in granules (Figure 4A). However, morphological changes in Hep-i(+) cells become more evident in Step 2. Cell-cell boundaries become clear due to bile canaliculi formation (Figure 4A). Hepatic differentiation can be confirmed by the expression of hepatic marker genes (Figure 4B) and proteins (Figure 4C) and hepatic functionality, such as glycogen storage (Figure 4D). CLiPs can also differentiate into BECs using a two-step protocol (Figure 5A). In Step 1, CLiPs are co-cultured on cell cycle-arrested mouse embryonic fibroblasts (MEFs) in mTeSR1 + YAC for 6 days. In Step 2, CLiPs are cultured for another 6 days in 2% Matrigel-containing mTeSR1 + YAC. CLiP-derived BEC-like cells form ductal and cystic structures typically observed in Step 2 (Figure 5B, Batch #1). However, batch-to-batch variability is noted. In some experiments, CLiPs start to form ductal/cystic structures in Step 1 (Figure 5B, Batch #2). CLiP-derived ductal/cystic structures express BEC marker proteins (Figure 5C) and exhibit the capacity to transport fluorescein to their luminal spaces following incubation with fluorescein diacetate (Figure 5D).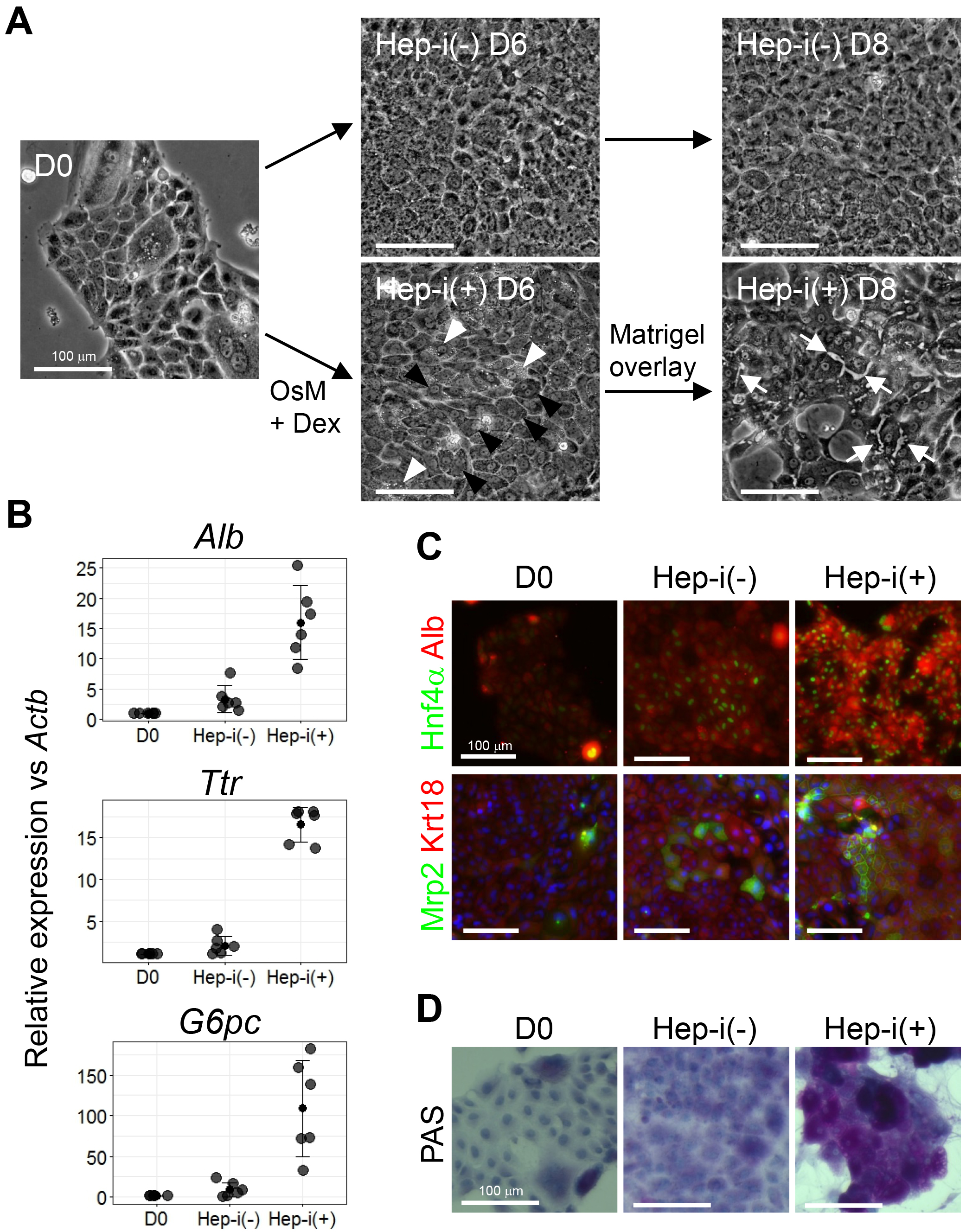
Figure 4. Characterization of CLiPs that underwent hepatic induction. A. Morphological change of CLiPs during hepatic induction. Black arrowheads indicate cells with decreased nucleus/cytoplasm ratio. White arrowheads indicate cells that are rich in granules in their cytoplasm. White arrows indicate bile canaliculi formed at the boundaries between cells that achieve hepatic maturation. B. qRT-PCR of hepatic marker genes for CLiPs before hepatic induction, Hep-i(-) and Hep-i(+) cells. The data represent the mean ± SD of 6 experiments performed with D14-16 CLiPs generated from 4 donor rats. C. ICC was performed for CLiPs before hepatic induction, Hep-i(-) and Hep-i(+) cells. Nuclei were counterstained with Hoechst 33342. The antibodies used for ICC have been previously described (Katsuda et al., 2017). D. The glycogen storage capacity was assessed by periodic acid-Schiff (PAS) staining using a commercially available staining kit (PAS kit). Nuclei were counterstained with hematoxylin.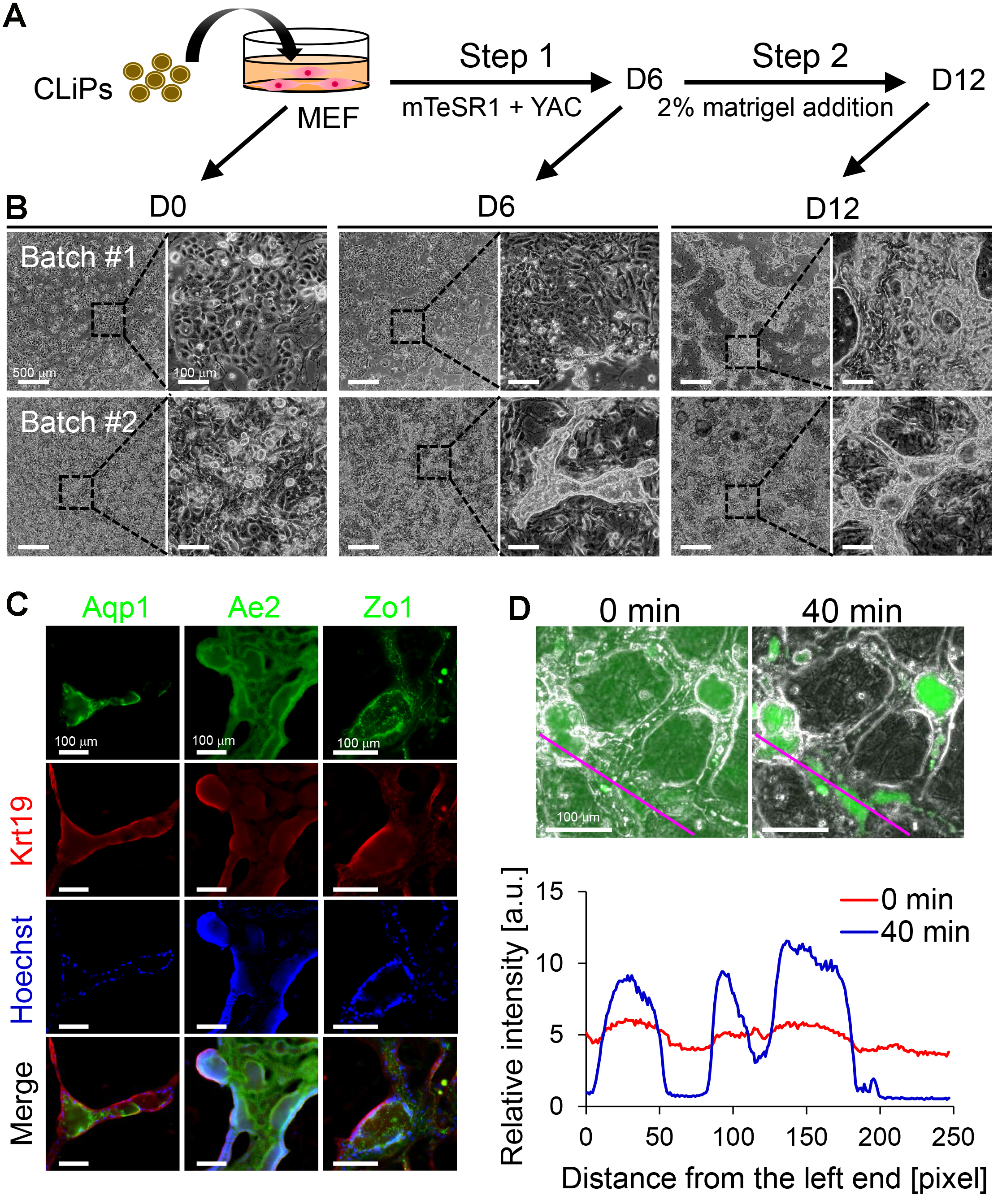
Figure 5. Characterization of CLiPs that underwent BEC induction. A. Schematic of the protocol for BEC induction from CLiPs. B. Morphological changes of CLiPs during BEC induction for 2 batches of experiments. CLiPs typically form ductal/cystic structures in Step 2 (Batch #1). In some experiments, CLiPs form ductal structures as early as Step 1 (Batch #2). C. CLiP-derived ductal/cystic structures express BEC markers, including Krt19, Aqp1 and Ae2, and the tight junction marker Zo1. D. CLiP-derived BEC-like cells exhibit the capacity to export fluorescein into their luminal space following incubation with fluorescein diacetate (FD). Phase-contrast and fluorescence images were immediately obtained after a 15-min incubation in the presence of FD (upper left panel) and an additional 40-min incubation in the absence of FD (upper right panel). The lower panel presents quantitative data of the fluorescence intensity profile along the magenta lines placed on the upper panels. The relative intensity is represented as the fluorescence intensity normalized with the total intensity along each line.
Establishment of stable CLiPs through continuous subculturing (Steps 35-36): Rat CLiPs undergo a temporal decrease in proliferative capacity between approximately passage 2 and passage 7 (Figure 6A). However, after this period, they proliferate stably. After 10 passages, a CLiP population seems to be relatively more homogeneous compared with that in earlier passages; thus, we regard stable CLiPs as the cells that undergo 10 passages. Different culture periods are required for CLiPs to reach 10 passages among experimental batches (74 days to 102 days, n = 6 biological replicates) and technical replicates (Figure 6A) (82 days to 124 days, 6 technical replicates).
We confirmed that CLiPs can proliferate on both Matrigel- and collagen-coated plates (Figure 6B), but the plating efficiency is increased when cultured on Matrigel-coated plates, thereby allowing efficient subculturing of CLiPs (Figure 6C). Thus, we recommend the usage of Matrigel-coated plates for subculturing of CLiPs.
We also confirmed that stable CLiPs, which have grown for more than 10 passages, can also undergo hepatic maturation (Figures 7B and 7C). However, we have not tried BEC induction of stable CLiPs. Stable CLiPs established from 2 of 3 donor rats exhibited biliary differentiation in vivo after transplantation into immunodeficient mice with chronic liver injury (Katsuda et al., 2017). Thus, these results suggest that CLiPs maintain bipotentiality at least partly even after serial passages.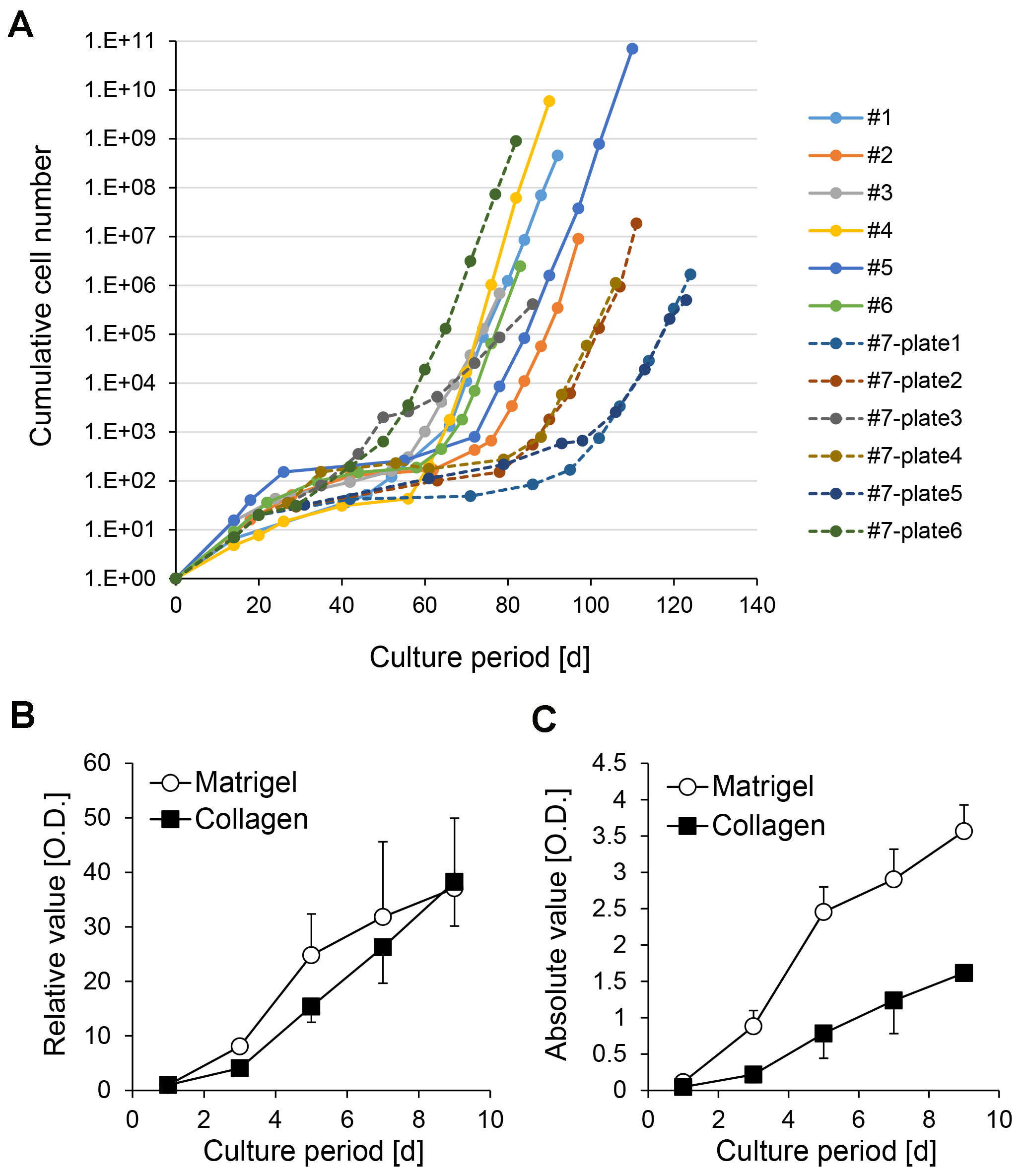
Figure 6. Inter-batch and intra-batch variation of growth rate of CLiPs during serial passages. A. Inter-batch variation of CLiP growth rates derived from 6 donor animals is presented as solid lines. The data from P0 to P9 or P10 of CLiPs are presented. Intra-batch variation of CLiP growth rate is presented for an additional donor animal (donor #7) as broken lines. Six plates (#7-plate1 to #7-plate6) were separately cultured for 10 passages. For #7 CLiPs, representative cellular numbers for P0 and P1 were determined from one plate. The cumulative cellular number was normalized to inoculated cell number in the primary culture. B. A stable CLiP cell line (#1) was seeded on collagen- or Matrigel-coated plates, and its proliferative capacity was assessed at the designated time points using the MTS assay. Signal intensity was normalized to the value of D1. The data represent the mean ± SE of 3 independent experiments. C. Absolute signal intensity for the data presented in (B). The data represent the mean ± SE of 3 independent experiments.
Limitations: As described above, the major limitation of this method is the difficulty experienced in its application to human hepatocyte reprogramming in its present form. We cultured cryopreserved human MHs in the presence of YAC and observed proliferation of epithelial cells (personal observation). These cells could be expanded. However, unlike rat and mouse MHs, they cease proliferation after approximately 4 passages. In addition, even early passage replicating cells did not exhibit hepatic differentiation capacity, suggesting that they are different from rodent CLiPs.
Another limitation is the imperfect reproducibility in the establishment of stable rat CLiPs with hepatic differentiation ability. Although the reproducibility of the induction of CLiPs from primary rat MHs is very rigid (100%, n > 50), obtaining stable CLiPs with the capacity of hepatic differentiation (namely, after 10 passages) appears to be stochastic. We have obtained stable CLiPs in all the experiments we have performed (n = 8 in Wistar rat; n = 1 in Lewis rat; n = 2 in Long Evans rat; n = 5 in Long Evans Agouti rat; n = 4 in Long Evans Cinnamon rat). However, in some of these experiments, we lost CLiPs with hepatic differentiation capacity after long-term culture. This is likely due to the heterogeneity of CLiPs. Early passage CLiPs are morphologically heterogeneous, but they become relatively homogeneous after long-term culture through the selection of limited numbers of cells in the population presumably during the period in which the proliferation rate is decreased (P2-P7). Due to this presumable selection process, CLiPs without hepatic phenotype become dominant in the culture in some experiment.
To enrich for high-quality CLiPs, namely, hepatic-competent CLiPs, the exploration of prospective surface marker(s) will be important, but we have not yet identified these markers. At present, to guarantee the securement of stable CLiPs with hepatic phenotype, we recommend preparing multiple plates or wells of culture in one experiment that are independently cultured. For example, we recommend splitting primary culture of MHs in SHM + YAC to multiple 60- or 100-mm dishes or 6- or 12-well plates and independently continue subculturing of each well/dish until CLiPs overcome the transient growth decrease (Figure 7A). In such an experiment, to reduce the labor involved in subculturing, we do not count cell numbers in every subculturing. Instead, we dilute the harvested cells at a ratio of 1:4 (during P1-P2) or 1:3-1:2 (during P3-P7, depending on the confluency of cultured wells) so that the plated cells are 20-50% confluent. When the cultured cells reach a stable growth phase, we only keep the cells that maintain LPC-like morphology (e.g., plate 1 in Figure 7A) and discard the cells without hepatic morphology (e.g., plate 3 in Figure 7A). Morphological differences can predict the hepatic differentiation capacity of stable CLiPs (Figures 7B and 7C). Figure 7 presents an example in which 3 of 6 plates (plates 1, 4 and 6) from one donor animal maintained a hepatic phenotype after 10 passages, whereas the other 3 plates (plates2, 3 and 5) exhibited a reduction in the hepatic phenotype.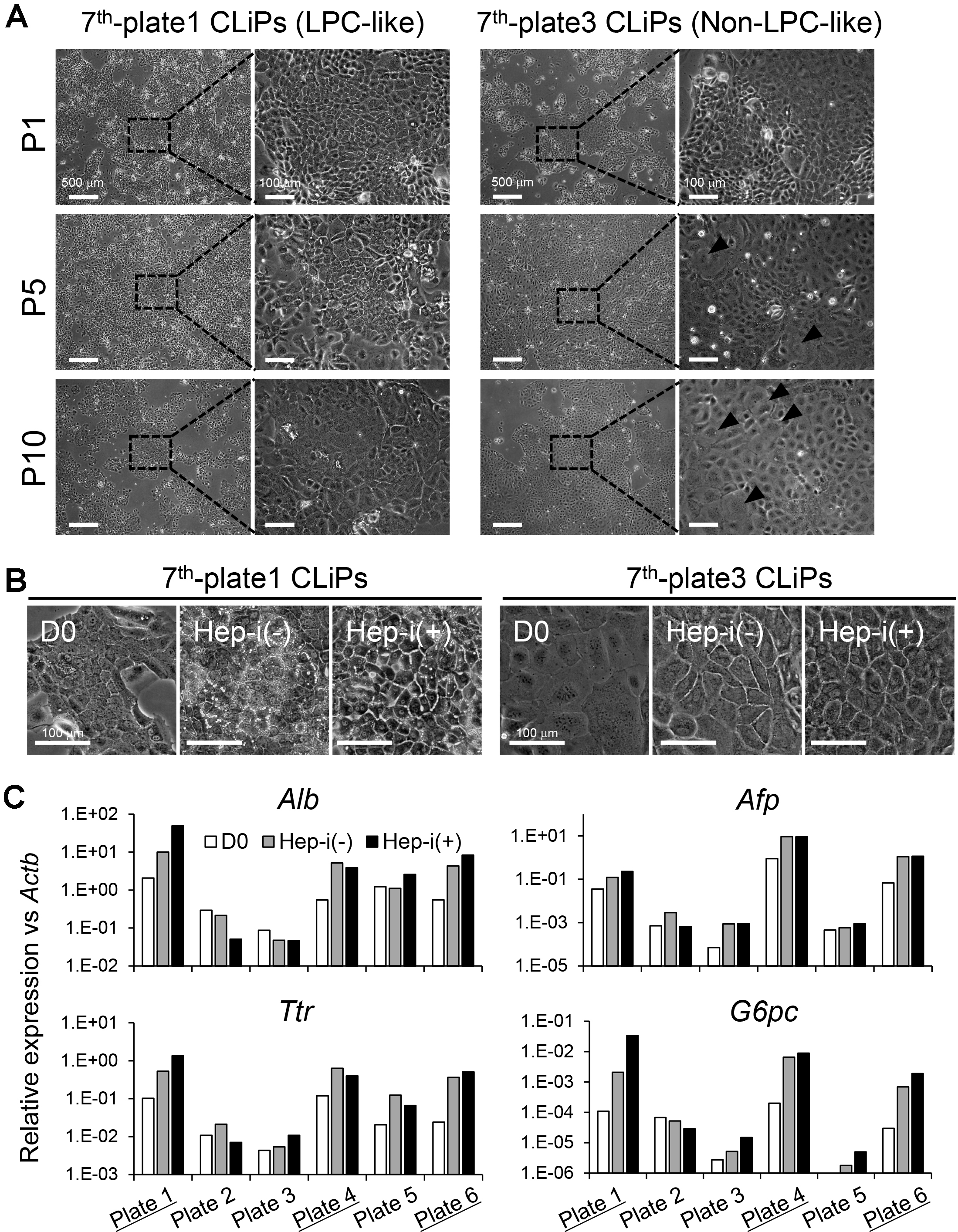
Figure 7. Morphological change of CLiPs during serial passages compared with non-LPC-like and LPC-like CLiPs. A. CLiPs cultured in two separate plates from one donor animal (donor #7) are photographed at P1, P4, P7 and P10. The morphology of non-LPC-like and LPC-like CLiPs is presented in the left and right columns, respectively. LPC-like CLiPs are small and exhibit a high nucleus/cytoplasm ratio. In contrast, non-LPC-like CLiPs are large and exhibit a relatively low nucleus/cytoplasm ratio. B. These non-LPC-like cells (plate 3) exhibit reduced responsiveness to hepatic induction. In contrast, LPC-like cells become MH-like cells with bile canaliculi-like structures following hepatic induction. C. qRT-PCR for hepatic marker genes for donor #7-derived CLiPs that were cultured individually in separate plates. CLiPs cultured in 3 of 6 plates exhibit increased hepatic maturation capacity (highlighted with underlines).
Materials and Reagents
- Paper towels
- Foam or cork board
- Silicon tube (Ø4.76 x 7.94 mm2) (TOKYO RIKAKIKAI, EYELA, catalog number: 125540 )
- Collagen type I-coated plate
96-well plate (IWAKI, catalog number: 4860-010 )
24-well plate (IWAKI, catalog number: 4820-010 )
12-well plate (IWAKI, catalog number: 4815-010 )
6-well plate (IWAKI, catalog number: 4810-010 )
100 mm dish (IWAKI, catalog number: 4020-010 ) - Non-coated tissue culture plate
96-well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 167008 )
24-well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 142475 )
6-well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675 )
100 mm dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 172931 ) - Sterilized cotton gauze (Osaki Medical Corporation, catalog number: 15095 )
- 100 ml centrifugation tube (IWAKI, catalog number: 2355-100 )
- 50 ml conical centrifuge tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339652 )
- Stainless 60 μm cell strainer (Ikemoto Scientific Technology, catalog number: 802-570-01 )
- Wide bored P200 tip (Thermo Fisher Scientific, catalog number: 118-96RNS )
- Cannula Ø1.2 mm (plastic outer needle equipped with intravenous cannula) (Top corporation, catalog number: 01151 )
- 4-0 Sterilized braid silk suture (Akiyama Medical MFG, catalog number: DEWB0404 )
- 0.22 μm filter (Stericup-GP), 250 ml (Merck, catalog number: SCGPU02RE )
- 25-ml pipette (Corning, Costar®, catalog number: 4489 )
- Wistar rats aged at 5 weeks to 20 weeks (both male and female animals can be used)
CAUTION: All animal experiments must comply with national and institutional regulations. - PAS kit (Sigma-Aldrich, catalog number: 395B-1KT )
- ISOFLURANE Inhalation Solution (Pfizer, catalog number: 871119 )
- Ethanol (Wako Pure Chemical Industries, catalog number: 054-07225 )
- E-MEM (Sigma-Aldrich, catalog number: M4655 )
- Percoll (GE Healthcare, catalog number: 17089102 )
- PBS(-) (Nissui, catalog number: 05913 )
- TrypLE Express (Thermo Fisher Scientific, GibcoTM, catalog number: 12604013 )
- Fetal bovine serum (FBS) (Gibco, catalog number not available)
Note: FBS should be heat inactivated at 56 °C for 30 min before use. - Cryopreserved MEFs (EmbryoMax® Primary Mouse Embryo Fibroblasts) (Merck, catalog number: PMEF-CF )
- CELLBANKER®1 Cell Freezing Media (Takara Bio, catalog number: CB011 )
- Liquefied nitrogen
- Sodium bicarbonate (NaHCO3) (Wako Pure Chemical Industries, catalog number: 191-01305 )
- Sodium chloride (NaCl) (Wako Pure Chemical Industries, catalog number: 192-13925 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Sodium phosphate monobasic dehydrate (NaH2PO4·2H2O) (Wako Pure Chemical Industries, catalog number: 192-02815 )
- Sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O) (Wako Pure Chemical Industries, catalog number: 196-02835 )
- Glucose (Sigma-Aldrich, catalog number: G6152 )
- EGTA (DOJINDO, catalog number: 346-01312 )
- EDTA (DOJINDO, catalog number: 345-01865 )
- Phenol red (Sigma-Aldrich, catalog number: P3532 )
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902 )
- Trypsin inhibitor (Sigma-Aldrich, catalog number: T9128 )
- Collagenase (Wako Pure Chemical Industries, catalog number: 032-22364 )
- L-proline (Sigma-Aldrich, catalog number: P0380 )
- Sodium hydroxide (NaOH) (Wako Pure Chemical Industries, catalog number: 197-02125 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9647 )
- Human epidermal growth factor (EGF) (Sigma-Aldrich, catalog number: E9644 )
- Dexamethasone (100 mg) (Wako Pure Chemical Industries, catalog number: 047-18863 )
- Ascorbic acid-2 phosphate (Asc2P) (Wako Pure Chemical Industries, catalog number: 013-12061 )
- Y-27632 (25 mg) (RHO/ROCK pathway inhibitor; Inhibits ROCK1 and ROCK2) (Wako Pure Chemical Industries, catalog number: 251-00514 )
- A-83-01 (10 mg) (Activin/NODAL/TGF-β pathway inhibitor; Inhibits ALK5, ALK4, and ALK7) (Wako Pure Chemical Industries, catalog number: 035-24113 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855 )
- CHIR99021 (25 mg) (WNT pathway activator; Inhibits GSK3) (Axon Medchem, catalog number: 1386 )
- DMEM/F12 containing 2.4 g/L NaHCO3 and L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 11320082 )
- DMEM, high glucose, pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11995065 )
- Antibiotic/antimycotic solution (Thermo Fisher Scientific, GibcoTM, catalog number: 15240062 )
- Insulin-transferrin-serine (ITS)-X (Thermo Fisher Scientific, GibcoTM, catalog number: 51500-056 )
- Nicotinamide (Sigma-Aldrich, catalog number: N0636 )
- Recombinant Mouse Oncostatin M (OsM) (25 μg) (R&D Systems, catalog number: 495-MO-025 )
- Matrigel (Corning, catalog number: 354230 )
- 0.5% (10x) trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 15400054 )
- mTeSRTM 1 (STEMCELL Technologies, catalog number: 85850 )
- Hydrochloric acid (HCl) (35-37%) (Wako Pure Chemical Industries, catalog number: 081-03475 )
- Leibovitz’s L-15 medium (with additives: sodium pyruvate; L-glutamine; phenol red) (Thermo Fisher Scientific, GibcoTM, catalog number: 11415064 )
- HBSS (10x), no calcium, no magnesium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 14185052 )
- 7.5% NaHCO3 solution (see Recipes)
- Pre-perfusion buffer (see Recipes)
- 0.05% phenol red solution (see Recipes)
- 0.05% collagenase solution (see Recipes)
- 2 M HEPES solution (see Recipes)
- L-proline stock solution (see Recipes)
- 5 N NaOH solution (see Recipes)
- 5% BSA stock solution (see Recipes)
- 10 μg/ml EGF stock solution (see Recipes)
- 10-4 M dexamethasone (see Recipes)
- 1 M nicotinamide (see Recipes)
- 100 mM Asc2P stock solution (see Recipes)
- Y-27632 (5 mM) (see Recipes)
- A-83-01 (2.5 mM) (see Recipes)
- CHIR99021 (15 mM) (see Recipes)
- Small hepatocyte medium (SHM) (see Recipes)
- 10 μg/ml mouse OsM stock solution (see Recipes)
- Matrigel stock (see Recipes)
- Matrigel-coated plate (see Recipes)
- 0.05% (1x) trypsin-EDTA (see Recipes)
- Hepatic induction medium for Step 1 (HIM-1) (see Recipes)
- Hepatic induction medium for Step 2 (HIM-2) (see Recipes)
- BEC induction medium for Step 1 (BIM-1) (see Recipes)
- BEC induction medium for Step 2 (BIM-2) (see Recipes)
- L-15-based medium (for complete Percoll solution) (see Recipes)
- Complete Perocll solution (see Recipes)
Equipment
- Surgical scissors (any company’s product should be fine)
- Surgical tweezer (any company’s product should be fine)
- Peristaltic pump (TOKYO RIKAKIKAI, EYELA, catalog number: RP-1000 )
- Small animal anesthetizer (Muromachi Kikai, catalog number: MK-A110 )
- Air tight chamber (Muromachi Kikai, catalog number: MK-TB1 )
- Bubble trap (prepared in-house)
- CO2 incubator (Panasonic Healthcare, catalog number: MCO-170AICUVH-PJ )
- Compact Tabletop Refrigerated Centrifuge (KUBOTA, model: Model 2800 )
- Hemocytometers (Improved Neubauer) (Sunlead Glass, catalog number: A126 )
- Micropipette (Gilson, catalog numbers: F123600 for P20; F123601 for P200; F123602 for P1000)
- Unit water bath Thermominder SDminiN (TEITEC, model: SDminiN, catalog number: 0068750-000 )
- Magnetic stirrer (any company’s product should be fine)
- 150 ml plastic storage bottle (Corning, catalog number: 431175 )
- Refrigerator (any company’s product should be fine)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Katsuda, T., Hosaka, K. and Ochiya, T. (2018). Generation of Chemically Induced Liver Progenitors (CLiPs) from Rat Adult Hepatocytes. Bio-protocol 8(2): e2689. DOI: 10.21769/BioProtoc.2689.
分类
细胞生物学 > 细胞工程 > 部分重编程
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












