- EN - English
- CN - 中文
Mutant Huntingtin Secretion in Neuro2A Cells and Rat Primary Cortical Neurons
Neuro2A细胞和大鼠原代皮层神经元中突变亨廷顿蛋白的分泌
发布: 2018年01月05日第8卷第1期 DOI: 10.21769/BioProtoc.2675 浏览次数: 8565
评审: Oneil G. BhalalaSalome Calado BotelhoKae-Jiun Chang

相关实验方案

SPPL2a抑制剂处理细胞后的CD74N端片段聚集的检测
Rubén Martínez-Barricarte [...] Jean-Laurent Casanova
2019年06月05日 6981 阅读

毛细管纳米免疫实验定量分析经CD138筛选的骨髓瘤细胞蛋白
Irena Misiewicz-Krzeminska [...] Norma C. Gutiérrez
2019年06月20日 6854 阅读
Abstract
Quantitative analysis of proteins secreted from the cells poses a challenge due to their low abundance and the interfering presence of a large amount of bovine serum albumin (BSA) in the cell culture media. We established assays for detection of mutant huntingtin (mHtt) secreted from Neuro2A cell line stably expressing mHtt and rat primary cortical neurons by Western blotting. Our protocol is based on reducing the amounts of BSA in the media while maintaining cell viability and secretory potential, and concentrating the media prior to analysis by means of ultrafiltration.
Keywords: Protein secretion (蛋白质分泌)Background
A number of proteins are secreted from the cell into the extracellular environment via various secretory pathways. These pathways include the conventional secretory pathway following ER-Golgi-plasma membrane route (Lee et al., 2004) and multiple unconventional pathways, such as lysosomal exocytosis, translocation across the plasma membrane and exosome and ectosome release (Zhang and Schekman, 2013). To study these pathways, it is often necessary to analyze proteins secreted from cultured cells into the media. Proteins may be secreted either in free form or in association with vesicular membrane structures such as ectosomes and exosomes (Zhang and Schekman, 2013). Whether these proteins are membrane-associated or not determines the type of approach used for their analysis. To isolate membrane-associated proteins, media is usually subjected to differential centrifugation procedure upon which the protein-containing membranes are sedimented, concentrated and purified from the constituents of the media, which allows for unhampered protein analysis (Momen-Heravi et al., 2013). However, the analysis of free-form proteins is a more challenging task (Chevallet et al., 2007). Two major obstacles are 1) the presence of substantial amounts of bovine serum albumin (BSA) in the serum or other supplements added to most cell culture media, which may mask the protein of interest; and 2) low abundance in the media of at least some of the secreted proteins, including mHtt. This protocol has been developed in order to study secreted mHtt and may be useful for analyzing other low-abundance, free-form proteins in the media.
Protocol optimization
To tackle the issue of excessive BSA, we first monitored cell viability in various media with partial or total reduction of BSA. In the case of Neuro2A cells stably expressing N-terminal 571 amino acids of mHtt with 72 glutamines (Neuro2A-mHtt cells), the optimal medium was Opti-MEM, which not only preserved viability of the cells, but also enhanced the secretion of mutant huntingtin (mHtt) as compared to other media, such as DMEM without supplemented fetal bovine serum (FBS) and HBSS (Figure 1). Moreover, we observed that applying conditioned Opti-MEM (Opti-MEM pre-incubated with naïve Neuro2A cells which do not express mHtt) dramatically enhanced the secretion of mHtt from Neuro2A-mHtt cells, possibly due to enrichment of the media with factors influencing secretion (Figure 1). However, primary cortical neurons were not viable in the presence of Opti-MEM, whereas they survived well in Neurobasal medium, regardless of the addition of BSA-containing B27 supplement (Table 1). Since high amounts of B27 supplement lead to the appearance of BSA accumulates in the region of the membrane where mHtt normally migrates (even when BSA was partially removed by immunoprecipitation) (Figure 2), we opted for Neurobasal media with reduced amount of B27 (0.2%).
To address the issue of low abundance of mHtt in the media, we compared several approaches to concentrate the media and thus enrich mHtt. While TCA precipitation of proteins and immunoprecipitation of mHtt from the media did not give satisfactory results, ultrafiltration of the media proved to be the method of choice for both Neuro2A cells and neurons. We were then able to detect mHtt by immunoblotting (Figure 1).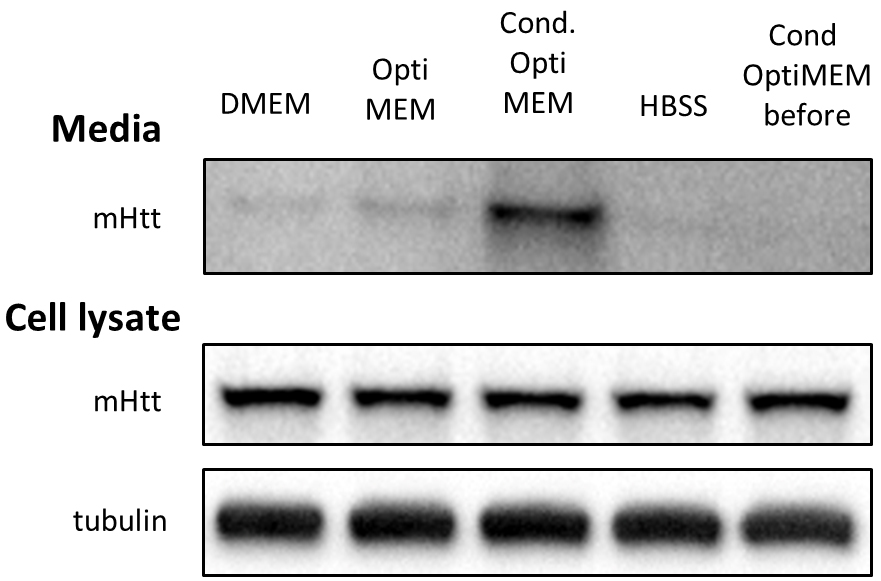
Figure 1. Optimizing conditions for detection of mHtt secreted from Neuro2A-mHtt cells. 800,000 mHtt-Neuro2A cells were incubated for 4 h in the following media: DMEM, Opti-MEM, conditioned Opti-MEM and HBSS. Cells were lysed, and media were collected, concentrated and analyzed by SDS-PAGE/immunoblotting using anti-Htt antibody. Tubulin was used as a loading control for the cell lysates. The fifth lane represents concentrated conditioned media before being applied on mHtt-expressing Neuro2A.
Table 1. Cells from Figure 2 were visually monitored for viability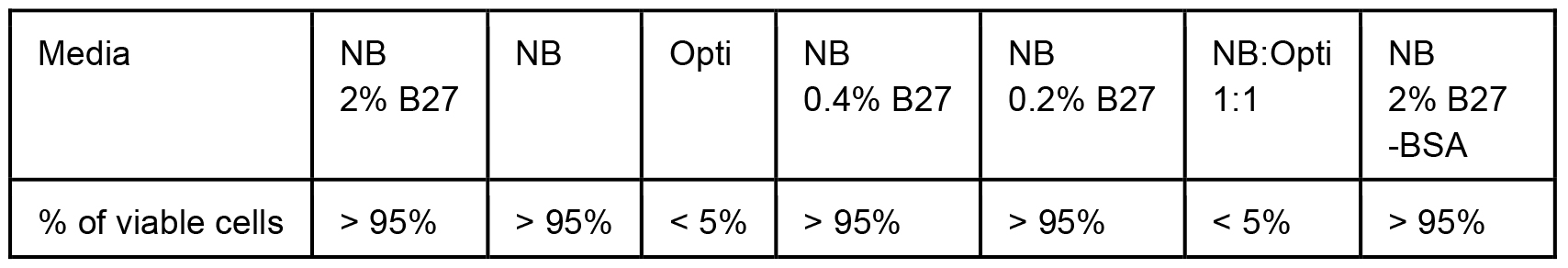
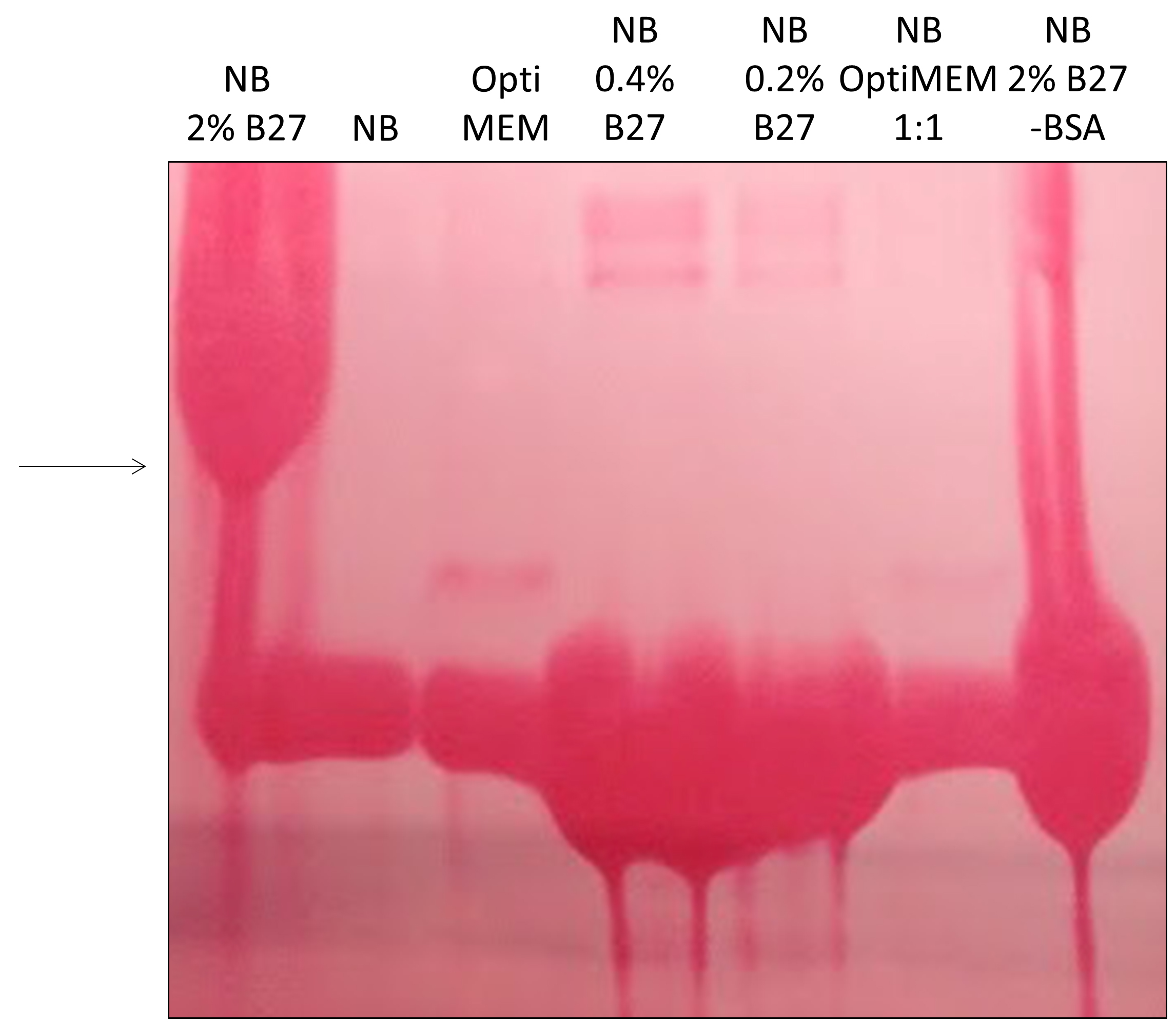
Figure 2. Optimizing conditions for detection of mHtt in primary cortical neurons. 700,000 cells were incubated overnight in Neurobasal media with 2% B27 supplement (NB 2% B27), plain Neurobasal media (NB), Opti-MEM, Neurobasal media with 0.4 or 0.2% B27, a mix of Opti-MEM and Neurobasal media at 1:1, or Neurobasal media with 2% B27 where BSA was partially depleted by immunoprecipitation using anti-BSA agarose beads (NB 2% B27 -BSA). The media were collected, concentrated and analyzed by SDS-PAGE/Western blotting. Membrane was stained by Ponceau S to reveal proteins. Large accumulations of proteins (BSA) in the region where mHtt normally migrates (arrow) revealed that the conditions for lanes 1 and 7 were not optimal for mHtt-immunodetection.
Materials and Reagents
- Pipette tips (Genesee Scientific, Olympus Plastics, catalog numbers: 24-412 , 24-430 , 24-401 )
- 100 mm dishes (Corning, catalog number: 430167 )
- 60 mm dishes (Corning, catalog number: 430166 )
- 12-well plates (Corning, catalog number: 3513 )
- 6-well plates (Corning, catalog number: 3516 )
- 1.5 ml centrifugation tubes (Fisher Scientific, Fisherbrand, catalog number: 05-408-129 )
- Amicon Ultra-0.5 centrifugal filter units, NMWL 10 (Millipore Sigma, catalog number: UFC501096 )
- 15 ml conical tubes (DOT Scientific, catalog number: 229411 )
- 50 ml conical tubes (DOT Scientific, catalog number: 229421 )
- Cell strainer (70 µm, Corning, Falcon®, catalog number: 352350 )
- NuPAGE® Novex® 3-8% Tris-Acetate Gels, 1.0 mm, 15-well (Thermo Fisher Scientific, InvitrogenTM, catalog number: EA03755BOX )
- Neuro2A cells (ATCC, catalog number: CCL-131 )
- Neuro2A-mHtt cells (stably expressing fragment of mHtt from amino acid 1-571 containing 72 glutamines) (Trajkovic et al., 2017)
- mHtt-Flag lentivirus (lentivirus encoding for a fragment of mHtt from amino acid 1-571 containing 72 glutamines with a C-terminal Flag tag)
- Sprague Dawley embryos E18, both sexes (Charles River Laboratories)
- Poly-D-lysine (Sigma-Aldrich, catalog number: P1149 )
- Boric acid (Sigma-Aldrich, catalog number: B7660 )
- Borax (Sigma-Aldrich, catalog number: B9876 )
- Sodium hydroxide (NaOH) (Avantor Performance Materials, catalog number: 5000-02 )
- DMEM (4.5 g/L Glucose, L-Glutamine, Sodium Pyruvate; Thermo Fisher Scientific, InvitrogenTM, catalog number: 11995073 )
- Opti-MEM (Thermo Fisher Scientific, InvitrogenTM, catalog number: 31985088 )
- Fetal bovine serum, certified, US origin (Thermo Fisher Scientific, InvitrogenTM, catalog number: 16000044 )
- 4x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 1610747 )
- 2x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 1610737XTU )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148 )
- Milk (Nestle Carnation instant nonfat dry milk)
- TBST (20x, with 2% Tween-20, pH 7.4) (Boston Bio Products, catalog number: IBB-180X )
- Anti-huntingtin antibody MAB5490 (Millipore Sigma, catalog number: MAB5490 )
- Peroxidase-AffiniPure Goat Anti-mouse IgG (H+L) (min X Hu, Bov, Hrs, Rb, Sw Sr Prot) (Jackson ImmunoResearch, catalog number: 115-035-146 )
- SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, catalog number: 34096 )
- 10x HBSS (Thermo Fisher Scientific, InvitrogenTM, catalog number: 14185052 )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- 100 mM sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360070 )
- D-glucose (Sigma-Aldrich, catalog number: G7021 )
- Penicillin-streptomycin, 10,000 U/ml (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15140122 )
- Trypsin 2.5% (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15090046 )
- Neurobasal media (Thermo Fisher Scientific, InvitrogenTM, catalog number: 21103049 )
- B27 supplement (50x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 17504044 )
- L-glutamine, 200 mM (Thermo Fisher Scientific, InvitrogenTM, catalog number: 25030081 )
- Ponceau S solution (Sigma-Aldrich, catalog number: P7170-1L )
- HIV-1 p24 Antigen ELISA (ZeptoMetrix, catalog number: 0 801111 )
- PierceTM LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific, catalog number: 88953 )
- 0.1 M borate buffer (see Recipes)
- DMEM/10% heat-inactivated FBS (see Recipes)
- 0.3 M HEPES (see Recipes)
- HBSS (see Recipes)
- Serum media (see Recipes)
- Full neuronal media (see Recipes)
- Neuronal secretion media (see Recipes)
Equipment
- Automatic pipettes (Gilson, model: Pipetman® L, catalog number: F167370 )
- FormaTM Steri-CycleTM CO2 Incubator, 37 °C (Thermo Fisher Scientific, model: FormaTM Steri-CycleTM )
- S-500 orbital shaker (VWR, model: S-500 , catalog number: 14005-830)
- Eppendorf® refrigerated centrifuge (Eppendorf, model: 5417 R )
- Allegra X-30 centrifuge (Beckman Coulter, model: Allegra® X-30 )
- Bio-Rad Trans-Blot® TurboTM Transfer System (Bio-Rad Laboratories, model: Trans-Blot® TurboTM Transfer System )
- ChemiDocTM XRS+ System (Bio-Rad Laboratories)
Software
- ImageJ software
- Image LabTM Software for ChemiDocTM XRS+ System (Bio-Rad Laboratories)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Trajkovic, K., Jeong, H. and Krainc, D. (2018). Mutant Huntingtin Secretion in Neuro2A Cells and Rat Primary Cortical Neurons. Bio-protocol 8(1): e2675. DOI: 10.21769/BioProtoc.2675.
- Trajkovic, K., Jeong, H. and Krainc, D. (2017). Mutant huntingtin is secreted via a late endosomal/lysosomal unconventional secretory pathway. J Neurosci 37(37): 9000-9012.
分类
神经科学 > 神经系统疾病 > 细胞机制
生物化学 > 蛋白质 > 免疫检测 > 免疫印迹法(WB )
细胞生物学 > 基于细胞的分析方法 > 蛋白质分泌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link