- EN - English
- CN - 中文
Easy and Efficient Permeabilization of Cyanobacteria for in vivo Enzyme Assays Using B-PER
使用B-PER简单有效地透化蓝藻以进行体内酶分析
发布: 2018年01月05日第8卷第1期 DOI: 10.21769/BioProtoc.2667 浏览次数: 7445
评审: Dennis NürnbergAnonymous reviewer(s)

相关实验方案
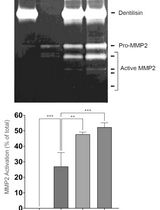
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2064 阅读
Abstract
Cyanobacteria are photosynthetic bacteria that thrive in diverse ecosystems and play major roles in the global carbon cycle. The abilities of cyanobacteria to fix atmospheric CO2 and to allocate the fixed carbons to chemicals and biofuels have attracted growing attentions as sustainable microbial cell factories. A better understanding of activities of enzymes involved in the central carbon metabolism might lead to increased product yields. Currently, cell-free lysates are widely used for the determination of intracellular enzyme activities. However, due to thick cell walls in cyanobacteria, lysis of cyanobacterial cells is inefficient and often laborious. The present protocol describes an easy and efficient method to permeabilize cyanobacterial cells, without lysing them, and direct usage of the permeabilized cells for the determination of metabolic enzyme activities in vivo.
Keywords: B-PER (B-PER)Background
We have previously reported an easy, efficient, and scalable permeabilization of cyanobacteria using the B-PERTM reagent (Thermo Fisher Science) (Rasmussen et al., 2016). The B-PERTM reagent contains a non-disclosed mild detergent dissolved in a Tris-HCl buffer and is typically used to lyse bacterial cells such as Escherichia coli. Serendipitously, we found that the B-PERTM reagent permeabilized cyanobacterial cells, instead of lysing them, likely because the thick cyanobacterial cell wall (Hoiczyk and Hansel, 2000) confers resistance to the detergent used in the reagent. Permeabilization was conducted in biotechnologically interesting cyanobacteria, Synechococcus sp. PCC 7002 (hereafter Synechococcus 7002) and Synechocystis sp. PCC 6803 (hereafter Synechocystis 6803). Briefly, incubation of cyanobacterial cells in the B-PERTM reagent for 10 min resulted in permeabilization of the cells as confirmed by the SYTOX Green staining. No significant change in cell shape and no major loss of intracellular proteins were observed during the treatment. Determination of the activity of two enzymes, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and glucose-6-phosphate dehydrogenase (G6PDH), which play imperative roles in the central carbon metabolism, was performed. When used directly in the assays, the permeabilized cells exhibited the enzyme activities that were comparable to or even higher than those detected for cell-free lysates. Moreover, the permeabilized cells could be stored at -20 °C without losing the enzyme activities. The permeabilization process and subsequent activity assays were successfully adapted to a 96-well plate system, allowing mid-to-high throughput characterization. The protocol may be readily adapted to studies of other cyanobacterial species and other intracellular enzymes. The protocol presented in this article provides a standardized step-by-step procedure adapted to a 10-ml culture of exponentially-growing cyanobacteria, although it can also be scaled up to a larger culture volume (~1 L) or down to as little as 200-µl cultures grown in a microtiter plate (Rasmussen et al., 2016).
Materials and Reagents
- Eclipse® pipette tips (Labcon, catalog numbers: 4-1011-260-000 for 20 µl tips, 4-1018-260-000 for 200 µl tips, 4-1019-260-000 for 1,200 µl tips)
- 1-cm light path cuvettes, polystyrene/polymethyl methacrylate (VWR, catalog number: 634-0676 )
- NuncTM 96-well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 249946 )
- 15-ml Falcon tubes (Greiner Bio One International, catalog number: 188271 )
- 1.5-ml Eppendorf safe-lock microtubes (Eppendorf, catalog number: 0030120086 )
- 1.5-ml Eppendorf microtubes (Eppendorf, catalog number: 0030125150 )
- 1.5-ml NalgeneTM screw cap microcentrifuge tubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 342800-0020 )
- A culture of Synechococcus 7002 grown on a plate of A+ medium (Stevens et al., 1973) containing 1.5% (w/v) BD BactoTM dehydrated agar (BD, BactoTM, catalog number: 214050 )
- A culture of Synechocystis 6803 grown on a plate of BG11 medium (Stanier et al., 1971) containing 1.5% (w/v) BD BactoTM dehydrated agar (BD, BactoTM, catalog number: 214050 )
- A+ medium, liquid (Stevens et al., 1973)
- BG11 medium, liquid (Stanier et al., 1971)
- B-PERTM bacterial protein extraction reagent (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 78248 )
- A 20 mM Tris-HCl buffer, pH 7.5
- UltraPureTM Tris hydrochloride [tris(hydroxymethyl)aminomethane hydrochloride] (Tris-HCl) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15506017 )
- 96% (v/v) ethanol (Plum, catalog number: 201104 )
- 1 M sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
- Micro BCATM protein assay kit (Thermo Fisher Scientific)
- cOmpleteTM protease inhibitor cocktail without ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, Roche Diagnostics, catalog number: 04693116001 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- Phosphocreatine (Sigma-Aldrich, catalog number: P7936 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- NADH (Sigma-Aldrich, Roche Diagnostics, catalog number: 10107735001 )
- Ribulose-1,5-bisphosphate (Sigma-Aldrich, catalog number: 83895 )
- Glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich, catalog number: G2267 )
- 3-Phosphoglyceric phosphokinase (Sigma-Aldrich, catalog number: P7634 )
- Creatine phosphokinase (Sigma-Aldrich, catalog number: C3755 )
- Tris(hydroxymethyl) aminoethane maleate (Sigma-Aldrich, catalog number: T3128 )
- NADP+ (Sigma-Aldrich, Roche Diagnostics, catalog number: 10128058001 )
- Glucose-6-phosphate (Sigma-Aldrich, Roche Diagnostics, catalog number: 10127647001 )
- cOmpleteTM protease inhibitor cocktail without ethylenediaminetetraacetic acid (EDTA) (see Recipes)
- Buffer A (see Recipes)
- A Rubisco assay buffer (see Recipes)
- G6PDH assay buffer (see Recipes)
Equipment
- Glass tubes (Inner diameter of 1.9 cm, max volume ~40 ml)
- P10 FinnpipetteTM F2 fixed volume single-channel pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642010 )
- P20 FinnpipetteTM F2 fixed volume single-channel pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642060 )
- P200 FinnpipetteTM F2 fixed volume single-channel pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642080 )
- P1000 FinnpipetteTM F2 fixed volume single-channel pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642090 )
- -20 °C freezer
- HiclaveTM autoclave (HMC Europe)
- Aquaria with temperature set at 30 °C for Synechocystis 6803 and 37 °C for Synechococcus 7002
- Philips Master TL-D, 18 W/840 cool white fluorescent tubes (Philips Lighting Holding, catalog number: 871150063171840 ). The number of fluorescence tubes is adjusted to achieve the photon flux of 50 µmol photons m-2 sec-1 for Synechocystis 6803 and 200 µmol photons m-2 sec-1 for Synechococcus 7002
- GMS150 gas mixer (Photon Systems Instruments, catalog number: GMS150 ) to provide 3% (v/v) CO2 balanced with air
- Holten horizontal laminar airflow sterile bench (Thermo Fisher Scientific, model: Holten Horizontal Laminar Airflow Clean Bench )
- QRT1 Quantitherm light meter/thermometer (Hansatech Instruments, model: QRT1 )
- Ultrospec 3100 pro UV/Visible spectrophotometer (GE Healthcare, Amersham Biosciences, model: Ultrospec 3100 pro )
- 2-L flask
- SorvallTM RC 6 Plus centrifuge (Thermo Fisher Scientific, model: SorvallTM RC 6 Plus , catalog number: 46910)
Note: This product has been discontinued. - PSU-20i multi-functional orbital shaking platform (Grant Instruments, model: PSU-20i )
- SpectraMax 190 microplate reader with SoftMax Pro software (Molecular Devices, model: SpectraMax 190 , catalog number: 190)
- Eppendorf ThermoMixer® C (Eppendorf, model: ThermoMixer® C , catalog number: 2231000269)
- Refrigerated tabletop centrifuge for 1.5 ml Eppendorf tubes (Thermo Fisher Scientific, Thermo ScientificTM, model: SorvallTM LegendTM Micro 17 , catalog number: 75002430)
Software
- Excel (Microsoft Office)
- SoftMax Pro software (see above for supplier)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Erstad, S. M. and Sakuragi, Y. (2018). Easy and Efficient Permeabilization of Cyanobacteria for in vivo Enzyme Assays Using B-PER. Bio-protocol 8(1): e2667. DOI: 10.21769/BioProtoc.2667.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link