- EN - English
- CN - 中文
Assessing Rates of Long-distance Carbon Transport in Arabidopsis by Collecting Phloem Exudations into EDTA Solutions after Photosynthetic Labeling with [14C]CO2
用[14C]CO2进行光合标记后通过EDTA溶液收集韧皮部渗出液来评估拟南芥长距离碳运输速率
发布: 2017年12月20日第7卷第24期 DOI: 10.21769/BioProtoc.2656 浏览次数: 6974
评审: Rebecca Van AckerAnonymous reviewer(s)
Abstract
Phloem loading and transport of photoassimilate from photoautotrophic source leaves to heterotrophic sink organs are essential physiological processes that help the disparate organs of a plant function as a single, unified organism. We present three protocols we routinely use in combination with each other to assess (1) the relative rates of sucrose (Suc) loading into the phloem vascular system of mature leaves (Yadav et al., 2017a), (2) the relative rates of carbon loading and transport through the phloem (this protocol), and (3) the relative rates of carbon unloading into heterotrophic sink organs, specifically roots, after long-distance transport (Yadav et al., 2017b), We propose that conducting all three protocols on experimental and control plants provides a reliable comparison of whole-plant carbon partitioning, and minimizes ambiguities associated with a single protocol conducted in isolation (Dasgupta et al., 2014; Khadilkar et al., 2016). In this protocol, [14C]CO2 is photoassimilated in source leaves and phloem loading and transport of photoassimilate is quantified by collecting phloem exudates into an EDTA solution followed by scintillation counting.
Keywords: Arabidopsis (拟南芥)Background
The allocation of reduced carbon and other compounds from photoautotrophic source tissues to heterotrophic sink tissues through the phloem is a crucial physiological process influencing growth and yield of plants. Because of this central role, there is interest in analyzing and quantifying phloem content from many areas of plant biology. However, collecting authentic phloem sap is difficult because the translocation stream is generally under high hydrostatic pressure and sieve elements have a rapid self-sealing mechanisms to prevent loss when damaged. Several collection techniques have emerged, but there is not currently a single or combination of methods that provide a complete and artifact-free measure of translocating phloem sap. Here, we briefly describe alternative techniques before detailing our approach to collecting phloem exudates into solutions containing low concentrations of ethylenediaminetetraacetic acid (EDTA) after photosynthetically labeling shoots with [14C]CO2. Turgeon and Wolf provide a comprehensive review of alternative techniques and their limitations (Turgeon and Wolf, 2009).
Phloem feeding insects, including aphids, scale insects, and planthoppers, evolved mechanisms to feed by directly drawing phloem sap from a plant’s vascular system and evade the self-sealing mechanism. Severing the feeding insect from a stylet penetrated into the phloem–referred to as stylectomy–can provide nanoliter to microliter quantities of sap that may most accurately reflect phloem content. Limitations of the technique are that it is technically challenging, works with only specific insect/plant combinations, the insects are selective for phloem with desired content, and insect saliva injected into the plant influences phloem content (Will et al., 2007; Hewer et al., 2011).
Another technique is to use plants that exude solution from cut stems without apparent sealing. Cucurbits, legumes, Ricinus communis, and some trees are well known for this and have become model systems for studying metabolites and signaling compounds in the phloem. However, the sap collected by this technique generally has low sugar concentrations, suggesting significant dilution and contamination from non-phloem sources and, particularly in the case of cucurbits, may be derived from specialized extrafascicular phloem elements rather than canonical fascicular phloem within vascular bundles (Zhang et al., 2010 and 2012).
The most common technique to sample phloem contents, and the one described in this protocol, is to collect phloem exudates from cut stems or petioles into solutions containing low concentrations of chelating agents, such as EDTA. Cations, particularly calcium (Ca2+), are involved in the rapid self-sealing mechanism of sieve tubes. Therefore, application of chelating agents to the cut ends of phloem-containing tissues limits sealing and permits phloem exudations for long periods from most, if not all, plants (King and Zeevaart, 1974; van Bel and Hess, 2008; Liu et al., 2012; Tetyuk et al., 2013). Although this method is most commonly used, it has important limitations and is not without controversy. Exudates are not collected directly, but are diluted into the EDTA solution during the collection period. This method therefore does not provide a measure of concentration, but rather a rate of exudation (i.e., unit of quantity per unit of time). As with the use of plants that naturally continue to exude, it is not clear how much the exudate is diluted by other sources in the plant or how much of the exudate is derived from the fascicular phloem involved in long distance translocation of photoassimilate in phloem sap. Enzymes, such as invertase from damaged cells, can enter the exudate solution and alter the profile of molecules exuded, as discussed at length in several articles (van Bel and Hess, 2008; Liu et al., 2012). A particularly important pitfall of this approach is that EDTA is toxic to cells and promotes membrane leakage which exacerbates contamination of the exudates with the contents of damaged cells and alteration of metabolite composition by leaked enzymes (Turgeon and Wolf, 2009). To minimize these impacts, we use the lowest EDTA concentration that still prevents sieve element sealing and as little tissue as possible is submersed in the EDTA solution (van Bel and Hess, 2008). In addition, we limit EDTA uptake via the xylem by conducting exudations in darkness and high humidity to promote stomatal closure and limit transpiration. Importantly, this procedure uses photosynthetic labeling of rosette leaves with [14C]CO2, and exudates are collected from cut petioles that have negligible photosynthetic activity and are well shaded by the mature rosette above. Therefore, counting 14C in the exudate solution is a quantitative representation of photoassimilate translocated from the leaves. When combined with other protocols (Yadav et al., 2017a and 2017b), this contributes to a reliable comparison of whole-plant carbon partitioning, and minimizes ambiguities associated with a single protocol conducted in isolation (Dasgupta et al., 2014; Khadilkar et al., 2016).
Materials and Reagents
- Labeling chambers derived from clear plastic deli containers (e.g., 16.5 cm long, 13 cm wide, and 5 cm deep ‘B16-Double Hot Dog Clamshell Case’ from Douglas Stephens Plastics, Paterson 640 NJ)
- Potting mixture (Sun Gro Horticulture, catalog number: Fafard 3B Mix ; or similar)
- 10 ml syringe (without a needle attached) (Luer-Lok Tip, BD, catalog number: 309604 )
- Dow Corning high vacuum grease
- Modeling clay (American Art Clay)
- Scotch Removable Mounting Putty
- 5 ml plastic syringe barrel and plunger (Luer-Lok Tip, BD, catalog number: 309646 )
- Syringe needle, 1.5-2.0 inches, 18 gauge (Monoject Needle, Covidien, catalog number: 1188818112 )
- High-humidity chambers derived from the clear plastic deli containers described above, lined with wet paper towel
- Petri dishes (100 x 25 mm) (Fisher Scientific, catalog number: FB0875711 )
- 24-well culture plates (Greiner Bio One International, catalog number: 662160 )
- Microcentrifuge tubes (Fisher Scientific, catalog number: 05-408-138 )
- Microcentrifuge tubes, screw cap with O-rings (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3464 )
- 20 ml scintillation vials (Fisher Scientific, catalog number: 12383317 )
- Double edge razor blades (PERSONNA brand) (Electron Microscopy Sciences, catalog number: 72000 )
- Plant material (e.g., Arabidopsis thaliana Col-0, control and experimental material); > 12 healthy plants for each treatment with 3 WT and 3 of each experimental plant in each labeling chamber
- Sodium bicarbonate [14C]NaHCO3 (MP Biomedicals; catalog number: 0117441H ; 40-60 mCi/mmol; 2 mCi/ml; 5 mCi; 185 MBq)
- Lactic acid (85%) (Fisher Scientific, catalog number: A162-500 )
- Soda lime (LI-COR, catalog number: 9964-090 ) loosely packed to fill a column (e.g., Bio-Rad Econo-Column, 1.5 x 20 cm; Bio-Rad Laboratories, catalog number: 7371522 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- Potassium hydroxide (KOH) for pH adjustment (Fisher Scientific)
- Ethanol absolute (Pharmco-AAPER, catalog number: 111000200 )
- Commercial bleach (Sodium hypochlorite, NaClO)
- Ecolume scintillation fluid (MP Biomedicals, catalog number: 0188247004 )
- 5 mM EDTA solution (see Recipes)
Equipment
- Environmental growth chambers for control and experimental plants
- Personal safety equipment: lab coat, nitrile gloves (or similar), and eye protection
- Lamp suitable for photosynthetic labeling, such as a 400 W metal halide lamp (SYLVANIA 64490 - 400 Watt - BT37 - Metal Halide)
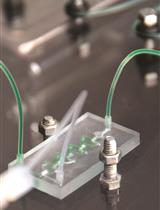
- Fume hood with appropriate support for metal halide lamp (Figure 1)
- Light meter (LI-COR 1400)
- Cork borer (The Science Company, catalog number: NC-11269 )
- Slim line micro blowers for air circulation (optional; Exton PA, Pelonis Technologies, catalog number: RFB3004 ; powered by four 1.5 volt D-cell batteries in sequence to provide 6 volts)
- Small air pump with inlet and outlet (e.g., Airpo, Barcodable, catalog number: UPC 045635496699 )
- Clear plastic tubing (4 and 6 mm internal diameter)
- Scissors (Fisher Scientific, catalog number: 08-951-20 )
- Forceps (Fisher Scientific, catalog number: 22-327379 )
- Balance with readability to at least 1 mg (METTLER TOLEDO, model: AE100 )
- Pipettes (Bioexpress, GeneMate, P20-P1000)
- Microcentrifuge
- Rotary platform shaker (Orbital Shaker Variable, BioExpress, GeneMate, catalog number: S-3200-LS )
- Geiger counter (Ludlum Measurements, model: Model 3 )
- Scintillation counter (Beckman Counter, model: LS 6000IC )
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yadav, U. P., Khadilkar, A. S., Shaikh, M. A., Turgeon, R. and Ayre, B. G. (2017). Assessing Rates of Long-distance Carbon Transport in Arabidopsis by Collecting Phloem Exudations into EDTA Solutions after Photosynthetic Labeling with [14C]CO2. Bio-protocol 7(24): e2656. DOI: 10.21769/BioProtoc.2656.
- Khadilkar, A. S., Yadav, U. P., Salazar, C., Shulaev, V., Paez-Valencia, J., Pizzio, G. A., Gaxiola, R. A. and Ayre, B. G. (2016). Constitutive and companion cell-specific overexpression of AVP1, encoding a proton-pumping pyrophosphatase, enhances biomass accumulation, phloem loading, and long-distance transport. Plant Physiol 170(1): 401-414.
分类
植物科学 > 植物生理学 > 营养
植物科学 > 植物生理学 > 光合作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link