- EN - English
- CN - 中文
In vitro Engineered DNA-binding Molecule-mediated Chromatin Immunoprecipitation (in vitro enChIP) Using CRISPR Ribonucleoproteins in Combination with Next-generation Sequencing (in vitro enChIP-Seq) for the Identification of Chromosomal Interactions
使用CRISPR核糖核蛋白的体外工程DNA结合分子介导的染色质免疫沉淀(体外enChIP)结合下一代测序(体外enChIP-Seq)用于染色体相互作用的鉴定
发布: 2017年11月20日第7卷第22期 DOI: 10.21769/BioProtoc.2612 浏览次数: 10420
评审: Jihyun KimXiao LiKate Hannan
Abstract
We have developed locus-specific chromatin immunoprecipitation (locus-specific ChIP) technologies consisting of insertional ChIP (iChIP) and engineered DNA-binding molecule-mediated ChIP (enChIP). Locus-specific ChIP is a method to isolate a genomic region of interest from cells while it also identifies what binds to this region using mass spectrometry (for protein) or next generation sequencing (for RNA or DNA) as described in Fujita et al. (2016a). Recently, we identified genomic regions that physically interact with a locus using an updated form of enChIP, in vitro enChIP, in combination with NGS (in vitro enChIP-Seq) (Fujita et al., 2017a). Here, we describe a protocol on in vitro enChIP to isolate a target locus for identification of genomic regions that physically interact with the locus.
Keywords: Chromatin immunoprecipitation (染色质免疫沉淀)Background
Elucidation of molecular mechanisms underlining genome functions requires the identification of molecules that interact with the genomic region of interest in vivo. To this end, we have developed locus-specific chromatin immunoprecipitation (locus-specific ChIP) technologies consisting of insertional ChIP (iChIP) and engineered DNA-binding molecule-mediated ChIP (enChIP) (Fujita et al., 2016a). Locus-specific ChIP is a method to biochemically isolate a genomic region of interest from cells. Molecules interacting with that isolated genomic region are identified by biochemical analyses, such as mass spectrometry (MS) and next generation sequencing (NGS). In iChIP, an exogenous DNA-binding protein and its recognition DNA sequence are used for ‘in cell’ locus-tagging. In enChIP, engineered DNA-binding molecules, such as transcription activator-like (TAL) proteins and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), are used for ‘in cell’ locus-tagging. The tagged loci are isolated by affinity-purification. We recently developed in vitro enChIP, in which the locus-tagging was performed in vitro (in a test tube) using recombinant and/or synthetic engineered DNA-binding molecules (Figure 1) (Fujita and Fujii, 2014a and 2016b). Here, we describe a protocol of in vitro enChIP using CRISPR ribonucleoproteins (RNPs) followed by NGS (in vitro enChIP-Seq) for the identification of genomic regions that physically interact with the locus of interest (Fujita et al., 2017a).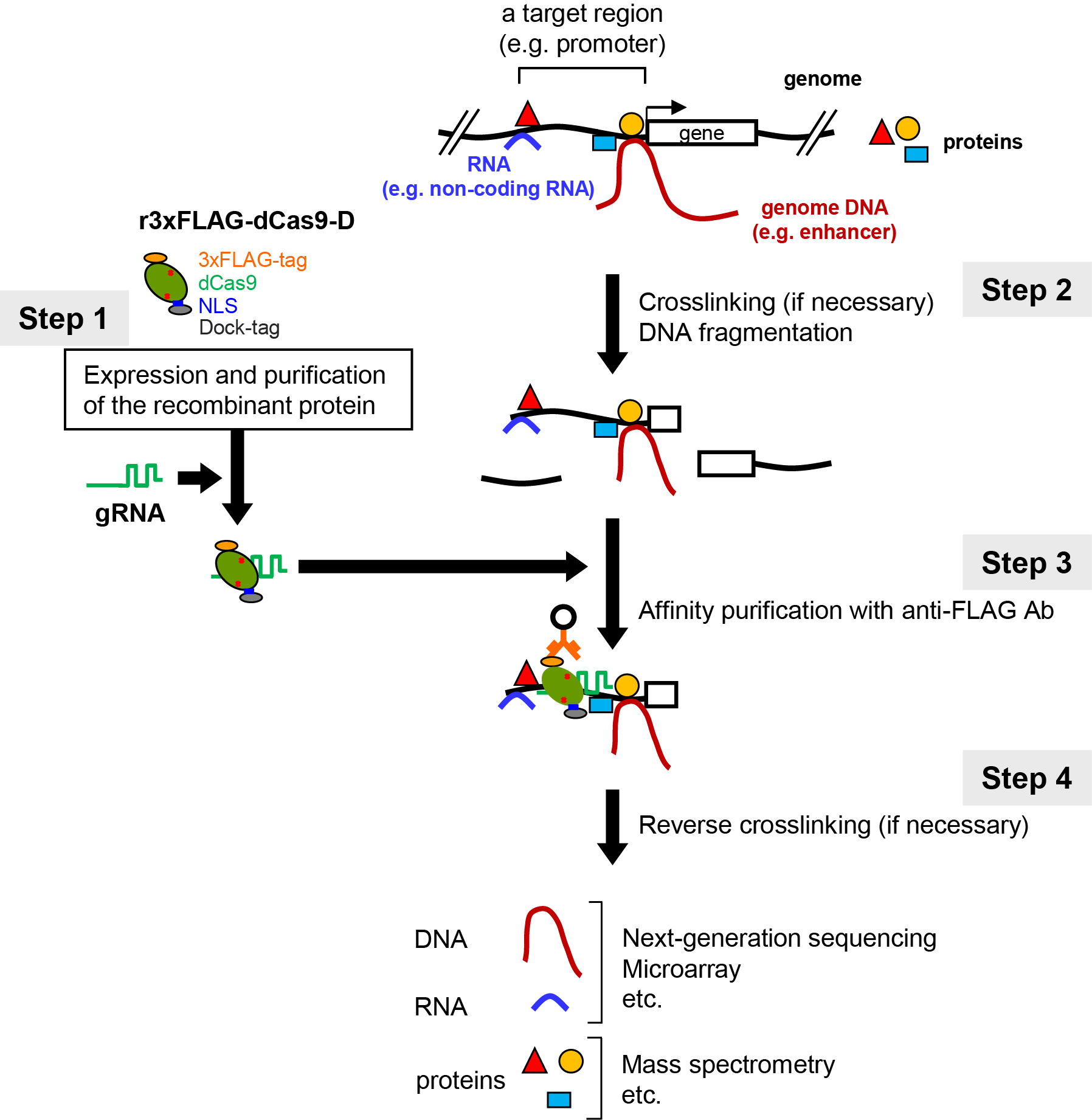
Figure 1. Scheme of in vitro enChIP using CRISPR RNPs. Figure 1 reproduced under the Creative Commons Attribution License from: Fujita et al. Efficient sequence-specific isolation of DNA fragments and chromatin by in vitro enChIP technology using recombinant CRISPR ribonucleoproteins. Genes Cells. 2016; 21: 370-377.
Scheme of in vitro enChIP using CRISPR RNPs (Figure 1)
1. A nuclease-dead form of Cas9 (dCas9) fused to an epitope-tag(s) (e.g., 3xFLAG-dCas9) is prepared as a recombinant protein. Guide RNA (gRNA) that recognizes the DNA sequence of the genomic region of interest is generated chemically. As to gRNA, single gRNA (sgRNA) or a complex of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) can be used.
2. Cells to be analyzed are crosslinked, if necessary, and lysed, and DNA is fragmented.
3. The 3xFLAG-dCas9/gRNA complex is incubated with the fragmented chromatin DNA in a test tube. The genomic DNA bound to the 3xFLAG-dCas9/gRNA complex is affinity-purified using antibody against the epitope-tag(s) or dCas9 itself. Alternatively, the complex can be purified using tags fused to gRNA (e.g., biotin). The isolated complexes retain molecules that interact with the target locus.
4. Reverse crosslinking, if necessary, and subsequent purification of DNA, RNA, or proteins allows identification and characterization of these molecules. For example, NGS or MS can be combined to identify genomic regions or proteins that physically bind to the target locus.
We previously described a detailed protocol of ‘in cell’ enChIP for the identification of proteins that interact with a locus of interest in Bio-protocol (Fujita and Fujii, 2014b). Procedures for the preparation of sonicated chromatin in in vitro enChIP are the same as those for ‘in cell’ enChIP. In addition, other procedures (e.g., preparation of Dynabeads) are similar between them. Therefore, we recommend to refer to the ‘in cell’ enChIP protocol (Fujita and Fujii, 2014b) in parallel.
Materials and Reagents
- 1.5 ml centrifuge tube (SARSTEDT, catalog number: 72.690.001 )
- Pipettes tips (DNase/RNase-free)
- DT40 cell (RIKEN BioResource Center) (an example)
- 10 µM crRNA (FASMAC, diluted in DNase/RNase-free water) (see step B1)
- 10 µM tracrRNA (FASMAC, diluted in DNase/RNase-free water) (see step B1)
- 3xFLAG-dCas9-D (Sysmex, ProCube)
- 37% formaldehyde (NACALAI TESQUE, catalog number: 16223-55 )
- Anti-FLAG M2 antibody (Sigma-Aldrich, catalog number: F1804 )
- Normal mouse IgG (Santa Cruz Biotechnology, catalog number: sc-2025 )
- Dynabeads-Protein G (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10004D )
- RNasin plus RNase inhibitor (Promega, catalog number: N261A )
- 10 mg/ml RNase A (Sigma-Aldrich, catalog number: R6513 )
- 10% SDS solution (NACALAI TESQUE, catalog number: 30562-04 )
- 20 mg/ml Proteinase K (Roche Diagnostics, catalog number: 3115828001 )
- ChIP DNA Clean and Concentrator (ZYMO RESEARCH, catalog number: D5205 )
- TruSeq ChIP Sample Prep Kit (Illumina, catalog number: IP-202-1012 )
- Potassium chloride (KCl) (NACALAI TESQUE, catalog number: 28538-62 )
- Dithiothreitol (DTT) (NACALAI TESQUE, catalog number: 14128-91 )
- 1 M HEPES (pH 7.1-7.5) (NACALAI TESQUE, catalog number: 17557-94 )
- 0.5 M ethylenediaminetetraacetate acid (EDTA) (pH 8.0) (NACALAI TESQUE, catalog number: 06894-85 )
- 1 M magnesium chloride hexahydrate (MgCl2·6H2O) (NACALAI TESQUE, catalog number: 20942-34 )
- cOmplete, Mini, EDTA-free Protease Inhibitor (Roche Diagnostics, catalog number: 4693159001 )
- UltraPure DNase/RNase-Free Distilled Water (DDW) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- 10x phosphate buffered saline (PBS) (pH 7.4) (NACALAI TESQUE, catalog number: 27575-31 )
- 1x PBS (10x dilution of 10x PBS with distilled water)
- Tween-20 (Sigma-Aldrich, catalog number: P5927 )
Note: This product has been discontinued. - BSA fraction V (7.5%) (Thermo Fisher Scientific, catalog number: 15260037 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9625 )
- 1 M Tris (pH 8.0) (AppliChem, catalog number: A4577 )
- 1 M Tris (pH 7.5) (AppliChem, catalog number: A4263 )
- Polyethylene Glycol Mono-p-isooctylphenyl Ether (Triton X-100) (NACALAI TESQUE, catalog number: 12967-45 )
- IGEPAL CA-630 (Sigma-Aldrich, catalog number: I8896 )
- 3xFLAG peptide (Sigma-Aldrich, catalog number: F4799 )
- 1 M KCl (see Recipes)
- 0.1 M DTT (see Recipes)
- In vitro CRISPR buffer (+ 0.5 mM DTT) (see Recipes)
- PBS-T (see Recipes)
- PBS-T-BSA (see Recipes)
- 5 M NaCl (see Recipes)
- Modified low salt buffer (+ 0.04% SDS) (see Recipes)
- TBS (see Recipes)
- TBS-IGEPAL CA-630 (see Recipes)
- Elution buffer (see Recipes)
Equipment
- Pipettes
- Ultrasonic Disruptor (TOMY SEIKO, model: UD-201 )
- Block heater (Eppendorf, catalog number: 5355 000.046 )
- Magnetic stand (Magical Trapper) (TOYOBO, catalog number: MGS-101 )
- Centrifuge (Eppendorf, catalog number: 5427 R )
- Vortex mixer (Fisher Scientific, catalog number: 128101 )
- Rotator (AS ONE, catalog number: TR-118 )
- HiSeq 2500 system (Illumina, model: HiSeq 2500 System )
Software
- Integrative Genomics Viewer, http://software.broadinstitute.org/software/igv/IGV
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Fujita, T. and Fujii, H. (2017). In vitro Engineered DNA-binding Molecule-mediated Chromatin Immunoprecipitation (in vitro enChIP) Using CRISPR Ribonucleoproteins in Combination with Next-generation Sequencing (in vitro enChIP-Seq) for the Identification of Chromosomal Interactions. Bio-protocol 7(22): e2612. DOI: 10.21769/BioProtoc.2612.
分类
分子生物学 > DNA > DNA 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link