- EN - English
- CN - 中文
Generation of Caenorhabditis elegans Transgenic Animals by DNA Microinjection
DNA显微注射法生成秀丽隐杆线虫转基因动物
发布: 2017年10月05日第7卷第19期 DOI: 10.21769/BioProtoc.2565 浏览次数: 15938
评审: Jyotiska ChaudhuriManish ChamoliAnonymous reviewer(s)
Abstract
Microinjection is the most frequently used tool for genetic transformation of the nematode Caenorhabditis elegans, facilitating the transgenic expression of genes, genome editing by the clustered regularly interspersed short palindromic repeats (CRISPR)-Cas9 system, or transcription of dsRNA for RNA intereference (RNAi). Exogenous DNA is delivered into the developing oocytes in the germline of adult hermaphrodites, which then generate transgenic animals among their offspring. In this protocol, we describe the microinjection procedure and the subsequent selection of transgenic progeny.
Keywords: Caenorhabditis elegans (秀丽隐杆线虫)Background
In C. elegans, DNA transformation by microinjection is commonly used to produce transgenic animals that over-express or ectopically express genes, which can be fused to tags (e.g., green fluorescent protein [GFP]), allowing for the phenotypic rescue of mutants, and/or the analysis of localization and function of proteins (Carter et al., 1990; Chalfie et al., 1994; Mello and Fire, 1995). The advent of the clustered regularly interspersed short palindromic repeats (CRISPR)-Cas9 system requires microinjection to achieve highly specific genome editing by introducing point mutations or insertion/deletion mutations (summarized in Dickinson and Goldstein, 2016). Further, the technique is applied for the inducible and/or tissue-specific in vivo transcription of dsRNA to facilitate heritable RNA interference (RNAi) (Tavernarakis et al., 2000). The genetic material is injected into the distal gonad syncytium of adult hermaphrodite animals where it enters the developing oocytes (Figure 1). The exogenous DNA is arranged into large extrachromosomal arrays consisting of 50 to 300 copies, which are inherited in a non-Mendelian fashion to the following generations. Next-generation progeny (F1) carry the extrachromosomal array with a varying probability (between 5 to 80% [Stinchcomb et al., 1985; Mello et al., 1991]). Transgenic animals are identified amongst the offspring by the use of co-transformation markers, which are injected along with the DNA of interest (Table 1). Most commonly used are the pharyngeal expression of green fluorescent protein (GFP) or red fluorescence (mCherry), or the dominant rol-6(su1006) allele, which induces a distinct rolling phenotype in transgenic F1 progeny (Mello et al., 1991; Tabara et al., 1996; Frokjaer-Jensen et al., 2008).
Table 1. Commonly used co-transformation markers with distinct phenotypes for selection of transgenic animals upon microinjection in C. elegans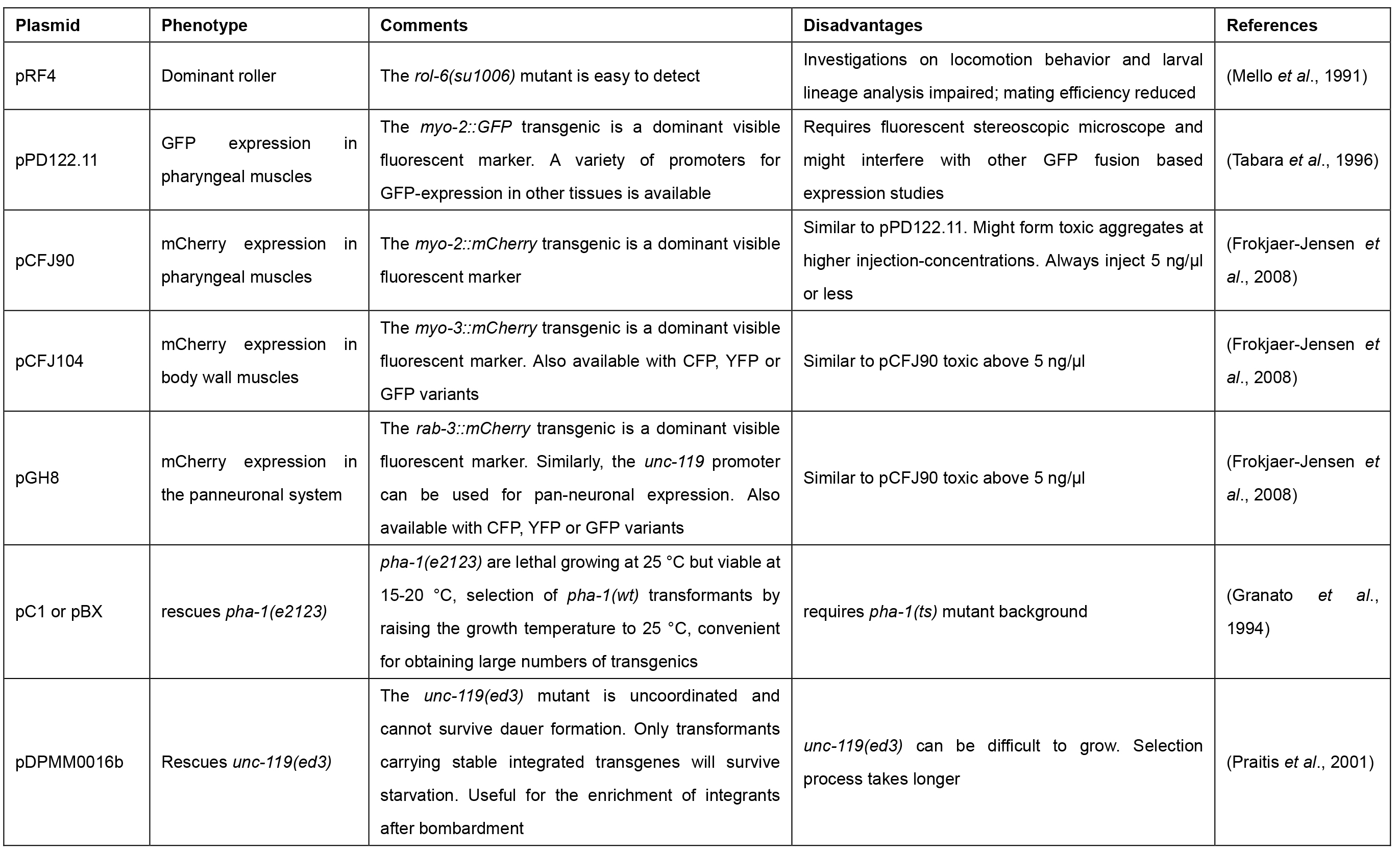
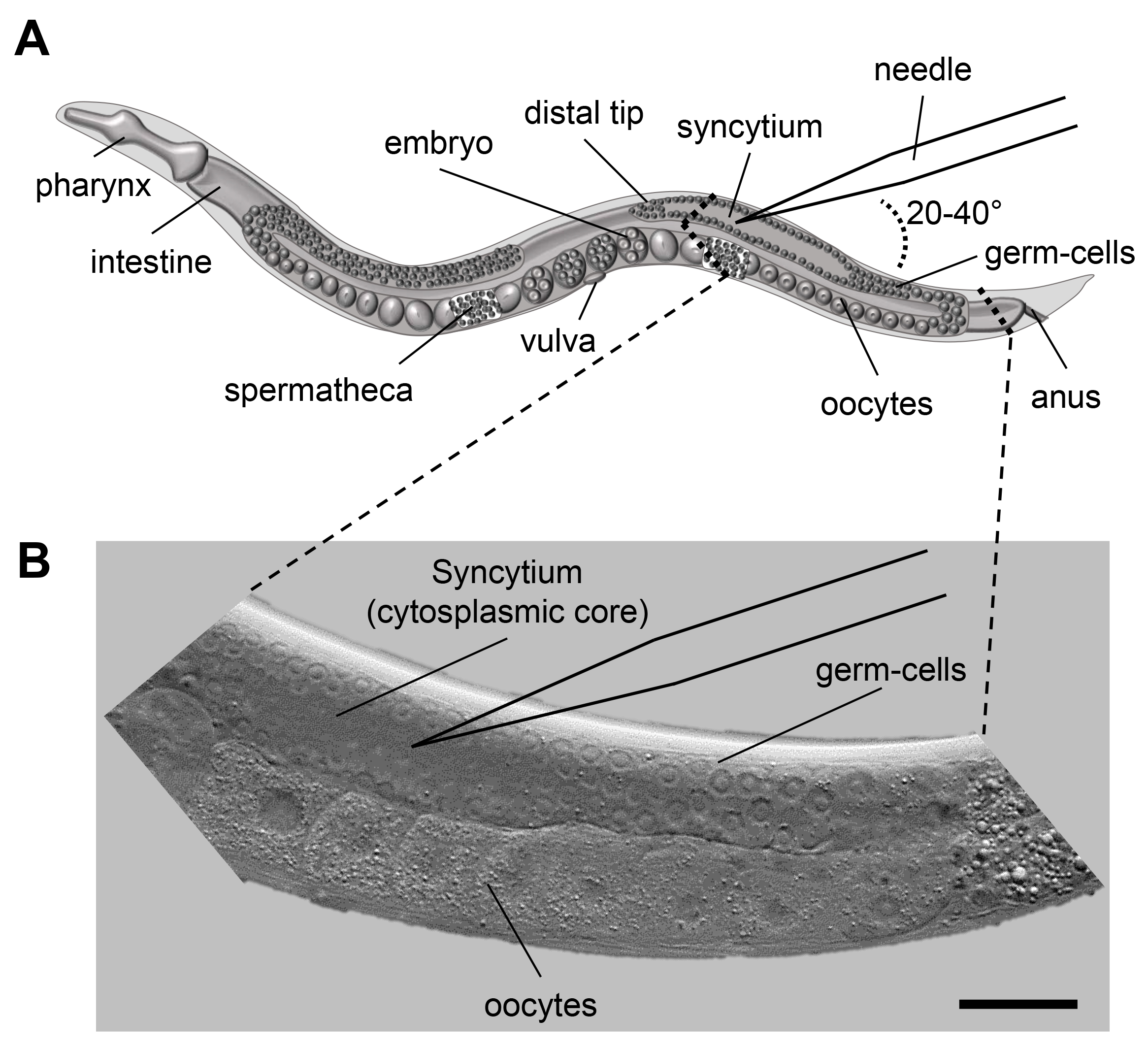
Figure 1. Microinjection scheme for C. elegans. A. Scheme of an adult C. elegans displaying the major organs including the pharynx, intestine and the gonad. When microinjecting C. elegans the injection capillary needs to be inserted in the syncytium (cytoplasmic core) of the distal gonad. The inlay indicates the area of interest for injection. B. DIC image of the area indicated in 1A. The nuclei of the germ-cells are clearly visible and surround the syncytium. Size bar corresponds to 50 µm.
Materials and Reagents
- Sterile pipette tips
- Microloader pipette tips (Eppendorf, catalog number: 5242956003 )
- Microinjection capillaries (Eppendorf Femtotips II, 0.5 µm inner and 0.7 µm outer diameter) (Eppendorf, catalog number: 930000043 )
- Microscope slides, 76 x 26 mm (Carl Roth, catalog number: 0656.1 )
- Microscope cover glasses, 24 x 60 mm (VWR, catalog number: 631-1575 )
- Tape (~1 mm thickness)
- Greiner Petri dishes (60 x 15 mm) (Sigma-Aldrich, catalog number: P5237 )
- Glass Pasteur Pipettes, disposable, 150 mm (BRAND, catalog number: 747715 )
- C. elegans animals (e.g., available from Caenorhabditis Genetics Center [CGC], University of Minnesota, MN, USA)
- Escherichia coli OP50 strain (obtained from the Caenorhabditis Genetics Center)
- DNA of interest for injection (plasmid, fosmid, PCR fragment, or similar) and plasmid containing a co-transformation marker (see Table 1)
- Miniprep Kit for DNA purification (e.g., QIAGEN-tip 20) (QIAGEN, catalog number: 10023 )
- Halocarbon oil 700 (Sigma-Aldrich, catalog number: H8898-100ML )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Peptone (BD, BactoTM, catalog number: 211677 )
- Streptomycin sulfate salt (Sigma-Aldrich, catalog number: S6501 )
- Agar (Sigma-Aldrich, catalog number: 05040 )
- 100% ethanol
- Calcium chloride dehydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C5080 )
- Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M7506 )
- Cholesterol stock solution (SERVA Electrophoresis, catalog number: 17101.01 )
- Nystatin stock solution (Sigma-Aldrich, catalog number: N3503 )
- Potassium dihydrogen phosphate (KH2PO4) (Carl Roth, catalog number: P018.1 )
- Di-Sodium hydrogen phosphate (Na2HPO4) (Carl Roth, catalog number: T876.1 )
- Di-Potassium hydrogen phosphate (K2HPO4) (Carl Roth, catalog number: 5066.1 )
- Agarose (Biozym Scientific, catalog number: 840004 )
- OP50 seeded NGM agar plates (see Recipes)
- 2% agarose pads (see Recipes)
- Hairpin pick (see Recipes)
- M9 buffer (see Recipes)
Equipment
- Bunsen burner
- Cylindrical glass beaker
- Wormpick with platinum wire and pre-flattened tip (Genesee Scientific, catalog numbers: 59-AWP and 59-30P6 )
- Microwave
- Heat plate (e.g., IKA, model: C-MAG HP 4 , catalog number: 0003581600)
- Puller (optional; e.g., Sutter Instrument, model: P-97 )
- Autoclave
- Stereomicroscope (e.g., Leica Microsystems, model: Leica M80 ), optionally fluorescent stereomicroscope (e.g., Leica Microsystems, model: Leica M165 FC)
- Microscope for Microinjection (ZEISS, model: Axio Observer.A1 equipped with differential interference contrast [DIC] prisms, gliding table, objective 10x/0.3 M27, objective 40x/0.75 M27, optionally camera AxioCam ERc 5 sec and monitor for observation and demonstration purposes)
- Micromanipulator (e.g., Eppendorf InjectMan 4) (Eppendorf, catalog number: 5192000019 )
- Microinjector unit (e.g., Eppendorf FemtoJet 4i) (Eppendorf, catalog number: 5252000013 )
- Microcentrifuge (e.g., Eppendorf Centrifuge 5424) (Eppendorf, model: 5424 , catalog number: 5404000014)
- Spectrophotometer (e.g., Thermo Fisher Scientific, model: NanoDropTM 8000 , catalog number: ND-8000-GL)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Rieckher, M. and Tavernarakis, N. (2017). Generation of Caenorhabditis elegans Transgenic Animals by DNA Microinjection. Bio-protocol 7(19): e2565. DOI: 10.21769/BioProtoc.2565.
分类
分子生物学 > DNA > 转化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














