- EN - English
- CN - 中文
GFP-Grb2 Translocation Assay Using High-content Imaging to Screen for Modulators of EGFR-signaling
通过高含量成像观测GFP-Grb2易位筛查EGFR信号转导调控因子
发布: 2017年09月05日第7卷第17期 DOI: 10.21769/BioProtoc.2528 浏览次数: 8651
评审: Ralph Bottcherilgen MenderTomas Aparicio

相关实验方案

用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2351 阅读
Abstract
High-content screening is a useful tool to understand complex cellular processes and to identify genes, proteins or small molecule compounds that modulate such pathways. High-content assays monitor the function of a protein or pathway by visualizing a change in an image-based readout, such as a change in the localization of a reporter protein. Examples of this can be the translocation of a fluorescently tagged protein from the cytoplasm to the nucleus or to the plasma membrane. One protein that is known to undergo such translocation is the Growth Factor Receptor-bound protein 2 (GRB2) that is recruited to the plasma membrane upon stimulation of a growth factor receptor and subsequently undergoes internalization. We have used GFP-tagged Grb2 previously to identify genes that are involved in EGFR signaling (Petschnigg et al., 2017). Ultimately, the assay can be adapted to cDNA expression cloning (Freeman et al., 2012) and can be used in early stage drug discovery to identify compounds that modulate or inhibit EGFR signaling and internalization (Antczak and Djaballah, 2016).
Keywords: Grb2 (Grb2)Background
Signal transduction by growth factor receptors is essential for cells to maintain proper function and thus requires tight control. Signal transduction by growth factor receptors is initiated by binding of an external ligand (e.g., Epidermal Growth Factor, EGF) to a transmembrane receptor such as the Epidermal Growth Factor Receptor (EGFR) and activation of downstream signaling cascades (Yao et al., 2015). A key regulator of EGFR-signaling is Growth Factor Receptor-bound protein 2 (Grb2), which is composed of an internal SH2 (Src homology 2) domain flanked by two SH3 domains. Grb2 binds to activated growth factor receptors at phosphorylated tyrosine residues through its SH2 domain, thus coupling receptor activation to SOS-Ras-MAPK (Mitogen-activated protein kinase) signaling cascades. The composition of Grb2 suggests that it can dock to a variety of receptors and transduce signals along multiple pathways. Mutations in signaling pathways frequently lead to the development of cancer. Hence, in order to better understand how aberrant signaling can lead to disease, it is important to identify novel signaling molecules in growth factor signaling. To accomplish this, we used the previously established microscopy-based GFP-Grb2 translocation assay that monitors the translocation of cytosolic GFP-tagged Grb2 to subcellular compartments upon expression of a cDNA library (Figure 1). We used this technique to identify novel proteins that can lead to translocation of GFP-Grb2 when overexpressed and in a second stage tested whether these proteins play a role in EGFR-signaling (Petschnigg et al., 2017). Examples that lead to punctate structures include TACC3, a novel EGFR-interactor, and AMPH (Figure 3). TACC3 led to induction of large GFP-Grb2 puncta, whereas AMPH results in formation of multiple small spots (Figure 3), pointing at potentially different mechanisms of those proteins in EGFR-signaling. In our recent study, we further characterized TACC3 and showed that TACC3 specifically binds to oncogenic EGFR variants and showed that TACC3 enhances EGFR-stability at the cell surface and increases EGFR-mediated signaling. The GFP-Grb2 assay can be expanded to multiple more applications. A siRNA/shRNA or CRISPR library could be co-expressed with GFP-Grb2 and translocation subsequently observed following EGF stimulation. As EGF stimulation would sequester GFP-Grb2 to endosomal structures and the plasma membrane, translocation from there upon siRNA/shRNA knockdown or CRISPR knockout could point at possible factors that ablate EGFR-Grb2 interactions and signaling. In a similar way, small molecule compound screens could be done to test for drugs that can specifically disrupt EGFR-Grb2 interactions upon EGF-stimulation (Figure 1). Other options could be to use mutated Grb2-variants that fail to bind to EGFR or other binding partners, thus the assay can give insight into which gene (when overexpressed or knock-downed) or which drug has an influence on specific binding domains of Grb2.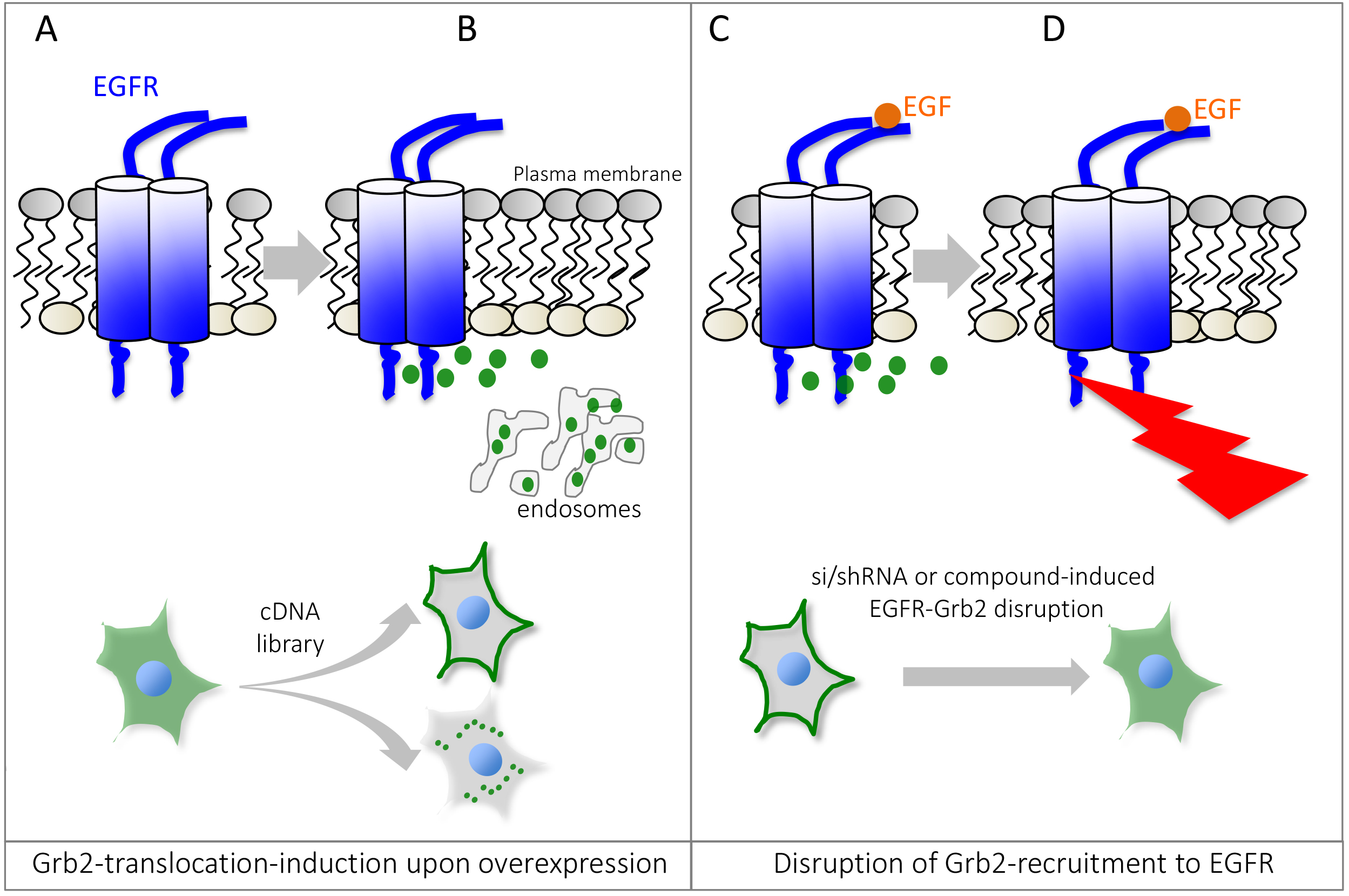
Figure 1. Schematic overview of the Grb2 translocation assay. Under non-stimulated or basal conditions, GFP-tagged Grb2 is mostly found in the cytosol (A), but can be recruited to localized interaction partners such as activated or endocytosed Epidermal Growth Factor Receptor, EGFR. Expression of a cDNA expression plasmid can lead to relocalization of GFP-Grb2 to the plasma membrane, endosomal structures or other sub-cellular locations by binding to Grb2 interaction partners or activation of Grb2-dependent cellular pathways (B). Stimulation with EGF recruits Grb2 to phosphotylated EGFR (C). Small molecule compounds or si/shRNA libraries can help identify genes or drugs that can disrupt Grb2 binding to the EGFR, impairing the recruitment to the receptor and thus predominant cytosolic localization of GFP-Grb2 (D). Since Grb2 can interact with other growth factor receptors as well, the assay can be adapted to monitor interaction/recruitment to other receptors as well.
Materials and Reagents
- Pipette tips (any standard sterile tips can be used, either non-filtered or filtered)
- ViewPlate-96 Black, Optically Clear Bottom (PerkinElmer, catalog number: 6005225 )
- Tissue culture plates: Nunc cell culture Petri dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 172931 )
- 0.22 µm filter
- HeLa cells (ATCC, catalog number: CCL-2 ) or HEK293T cells (ATCC, catalog number: CRL-3216 )
- pMOS-GFP-Grb2 plasmid (Ketteler et al., 2002)
- 3xFLAG-TACC3 plasmid (Petschnigg et al., 2017)
- Dulbecco modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 61965026 )
Note: Any commercially available DMEM can be used, and GlutaMax can be added, but is not required for HeLa and HEK293T cells. - Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10500056 )
- Penicillin-streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Trypsin/EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: R001100 )
- GlutaMax (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Paraformaldehyde, 4% (v/v) (Santa Cruz Biotechnologies, catalog number: sc-281692 )
Note: Should be stored at -20 °C in aliquots for long-term storage. Thawed aliquots can be kept at 4 °C for up to a month. - Hoechst 33342 trihydrochloride, trihydrate, 1 mg/ml stock (Thermo Fisher Scientific, InvitrogenTM, catalog number: H3570 )
- Polyethylenimine (PEI), 10 mg/ml stock in water (Sigma-Aldrich, catalog number: 408727 )
- Sodium chloride (NaCl), AnalaR NORMAPUR (VWR, catalog number: 27810.295 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Sodium phosphate dibasic (Na2HPO4), AnalaR NORMAPUR (VWR, catalog number: 102494C )
- Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P8281 )
- PEI solution (see Recipes)
- 1x phosphate buffered saline (PBS) (pH 7.4) (see Recipes)
- 10x phosphate buffered saline (PBS) (see Recipes)
Equipment
- Multi-channel pipette (Finnpipette, 8-channel P300, Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4661030N )
- Incubator (Eppendorf, model: Galaxy® 170 R )
- High-content screening microscope (PerkinElmer, Opera)
Software
- ImageJ
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Petschnigg, J. and Ketteler, R. (2017). GFP-Grb2 Translocation Assay Using High-content Imaging to Screen for Modulators of EGFR-signaling. Bio-protocol 7(17): e2528. DOI: 10.21769/BioProtoc.2528.
分类
癌症生物学 > 癌症生物化学 > 蛋白质
细胞生物学 > 细胞染色 > 蛋白质
细胞生物学 > 细胞信号传导 > 胞内信号传导
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










