- EN - English
- CN - 中文
Expression and Purification of the Cas10-Csm Complex from Staphylococci
表达并纯化葡萄球菌的Cas10-Csm复合体
发布: 2017年06月05日第7卷第11期 DOI: 10.21769/BioProtoc.2353 浏览次数: 12009
评审: Lionel SchiavolinLongping Victor TseDaan C. Swarts

相关实验方案
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2064 阅读
Abstract
CRISPR-Cas (Clustered regularly interspaced short palindromic repeats-CRISPR-associated proteins) is a class of prokaryotic immune systems that degrade foreign nucleic acids in a sequence-specific manner. These systems rely upon ribonucleoprotein complexes composed of Cas nucleases and small CRISPR RNAs (crRNAs). Staphylococcus epidermidis and Staphylococcus aureus are bacterial residents on human skin that are also leading causes of antibiotic resistant infections (Lowy, 1998; National Nosocomial Infections Surveillance, 2004; Otto, 2009). Many staphylococci possess Type III-A CRISPR-Cas systems (Marraffini and Sontheimer, 2008; Cao et al., 2016), which have been shown to prevent plasmid transfer and protect against viral predators (Goldberg et al., 2014; Hatoum-Aslan et al., 2014; Samai et al., 2015) in these organisms. Thus, gaining a mechanistic understanding of these systems in the native staphylococcal background can lead to important insights into the factors that impact the evolution and survival of these pathogens. Type III-A CRISPR-Cas systems encode a five-subunit effector complex called Cas10-Csm (Hatoum-Aslan et al., 2013). Here, we describe a protocol for the expression and purification of Cas10-Csm from its native S. epidermidis background or a heterologous S. aureus background. The method consists of a two-step purification protocol involving Ni2+-affinity chromatography and a DNA affinity biotin pull-down, which together yield a pure preparation of the Cas10-Csm complex. This approach has been used previously to analyze the effects of mutations on Cas10-Csm complex integrity (Hatoum-Aslan et al., 2014), crRNA formation (Hatoum-Aslan et al., 2013), and to detect binding partners that directly interact with the core Cas10-Csm complex (Walker et al., 2016). Importantly, this approach can be easily adapted for use in other Staphylococcus species to probe and understand their native Type III-A CRISPR-Cas systems.
Keywords: CRISPR-Cas Type III-A (CRISPR-Cas Type III-A)Background
Staphylococcus epidermidis and Staphylococcus aureus are prevalent skin-dwelling bacteria that have a range of opposing impacts. While S. aureus asymptomatically colonizes ~30% of the population (Conlan et al., 2012), this organism is a leading cause of skin and soft tissue infections (Stryjewski and Chambers, 2008; Grice and Segre, 2011). In contrast, S. epidermidis is generally considered beneficial, and promotes human health by 1) preventing S. aureus colonization (Iwase et al., 2010), 2) producing antimicrobial peptides that target skin pathogens (Cogen et al., 2010), and 3) stimulating the human immune system to facilitate pathogen defense (Lai et al., 2010; Naik et al., 2015). However, when allowed to breach the skin barrier, this species can also cause antibiotic resistant infections, particularly on indwelling medical devices (Otto, 2009; Harris and Richards, 2006). Furthermore, pathogenic staphylococci that are resistant to all known antibiotics have recently emerged in both hospital and community settings (Furuya and Lowy, 2006) and have become a major threat to global public health. Horizontal gene transfer (HGT), or the exchange of genetic information between related bacterial species, is a major route by which these organisms acquire virulence factors and multi-drug resistance. Therefore, it is of utmost importance to understand the factors that impact and regulate HGT in these organisms.
CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR associated proteins) is a class of bacterial immune systems that degrade invading nucleic acids and prevent all modes of HGT (Marraffini, 2015). CRISPR loci consist of short sequences derived from past invaders, known as spacers, which are integrated between repeat sequences of similar length (~30-40 nucleotides). These repeat-spacer arrays encode small CRISPR RNAs (crRNAs) that associate with Cas proteins, forming a ribonucleoprotein complex that destroys foreign DNA and/or RNA in a sequence-dependent manner. Many staphylococci possess Type III-A CRISPR-Cas systems (Marraffini and Sontheimer, 2008; Golding et al., 2012; Cao et al., 2016). The Type III-A system in S. epidermidis RP62a, a wild-type human isolate (Christensen et al., 1987), encodes a multi-subunit complex called Cas10-Csm, composed of Cas10, Csm2, Csm3, Csm4, Csm5 and a crRNA (Hatoum-Aslan et al., 2013). This system has been shown to prevent the conjugative transfer of antibiotic resistance genes (Marraffini and Sontheimer, 2008; Hatoum-Aslan et al., 2014) and phage infection (Goldberg et al., 2014; Maniv et al., 2016), thus providing a natural barrier for HGT, and a model for Type III CRISPR-Cas systems in staphylococci.
The overexpression and purification of recombinant CRISPR-associated proteins from Escherichia coli (both the Cas10-Csm complex and individual subunits) followed by in vitro biochemical assays have revealed important insights into their functions (Hatoum-Aslan et al., 2013; Samai et al., 2015; Walker et al., 2016). However, such assays fail to 1) recover information about protein function and stability in the native cellular environment, and 2) identify biologically relevant binding partners that are not a part of the core Cas10-Csm complex. Indeed, purification of Cas10-Csm from its native S. epidermidis background has yielded additional insights into the genetic requirements for complex stability and function, crRNA processing, and non-Cas binding partners that might play a role in the CRISPR-Cas pathway (Hatoum-Aslan et al., 2013 and 2014; Walker et al., 2016). Here, we provide a detailed protocol for the purification of Cas10-Csm from S. epidermidis or S. aureus strains bearing the Type III-A CRISPR-Cas system on a plasmid. The protocol involves two affinity-purification steps that can be carried out over the course of five days (Figure 1). Importantly, this protocol can be easily adapted to study Cas10-Csm complexes in other Staphylococcus species, thus providing an essential tool to probe and understand these important immune systems.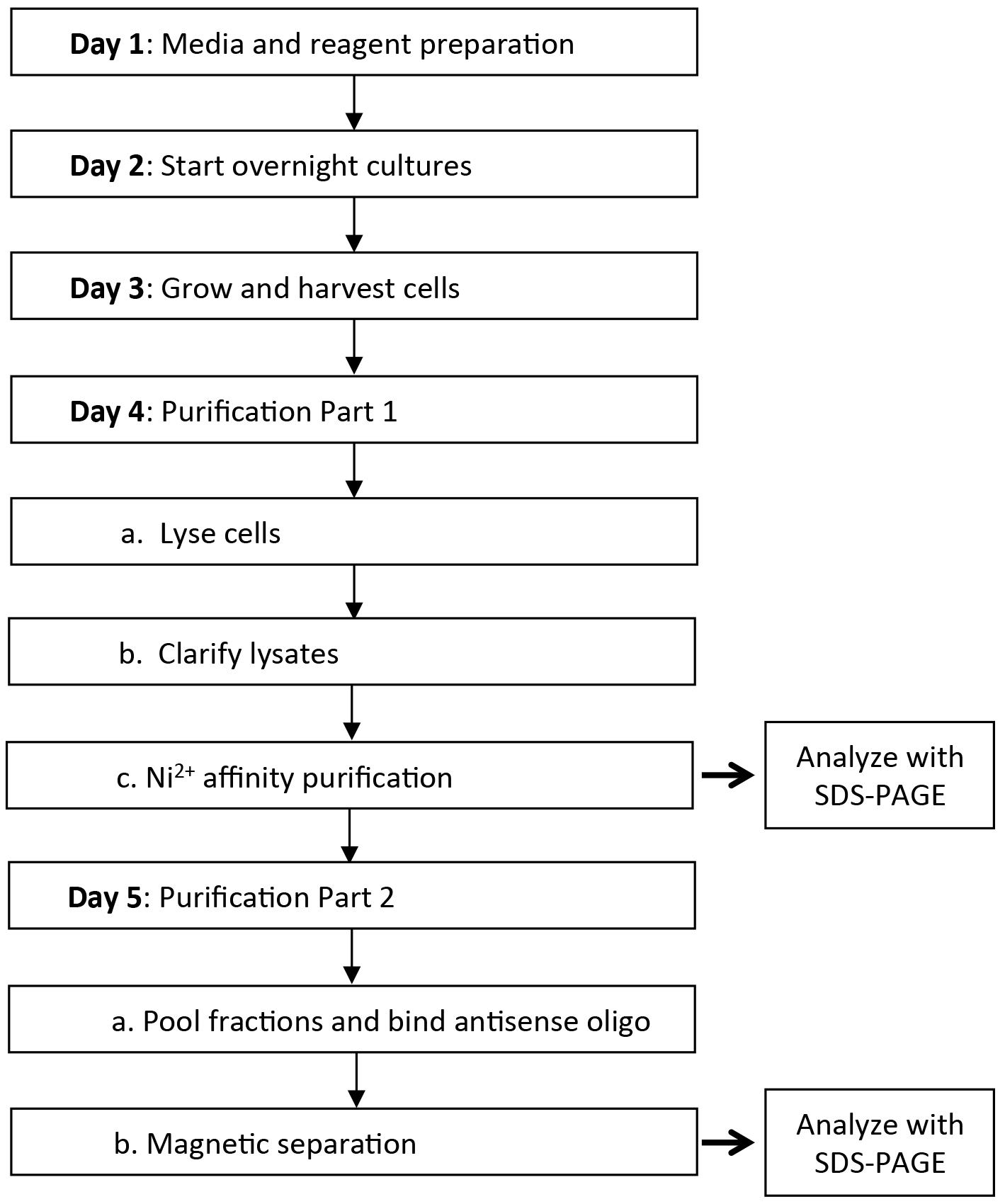
Figure 1. Timeline of activities for expression and purification of the Cas10-Csm complex from staphylococci
Materials and Reagents
Note: Equivalent materials and reagents may be used as substitutes.
- Centrifuge tubes (50 ml) (VWR, catalog number: 21008-242 )
- Pipet tips with filter (0.1-10 µl) (VWR, catalog number: 89368-972 )
- Pipet tips with filter (1-200 µl) (VWR, catalog number: 89003-056 )
- Pipet tips with filter (100-1,000 µl) (VWR, catalog number: 89003-060 )
- PES membrane vacuum filter (0.22 µm) (VWR, catalog number: 10040-468 )
- Centrifuge tubes (15 ml) (VWR, catalog number: 21008-216 )
- Microcentrifuge tubes (VWR, catalog number: 87003-294 )
- Cellulose syringe filter (0.22 µm) (VWR, catalog number: 28145-477 )
- Petri dishes (100 x 15 mm) (VWR, catalog number: 25384-088 )
- Spectrophotometer cuvettes (VWR, catalog number: 97000-586 )
- Syringe (10 ml) (BD, catalog number: 309604 )
- Syringe (3 ml) (BD, catalog number: 309657 )
- Centrifugation polypropylene bottles (400 ml) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 75007585 )
- Disposable gravity flow columns for protein purification (Geno Technology, G-Biosciences, catalog number: 786-169 )
- S. epidermidis LM1680 expressing pcrispr/Csm26HN (Hatoum-Aslan et al., 2013) (see Note 1)
- S. aureus RN4220 expressing pcrispr/Csm26HN (Hatoum-Aslan et al., 2013) (see Note 1)
- HisPur Ni-NTA resin (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88222 )
- Sera-MagTM magnetic streptavidin coated beads (GE Healthcare, catalog number: 30152105011150 )
- SDS PAGE Gel 0.75 MM ‘Snap-A-GelsTM Mini Tris Glycine Precast Gels, Jule’ (VWR, catalog number: 66025-389 )
- Color protein standard ladder (New England Biolabs, catalog number: P7712S )
- BBLTM brain heart infusion (BHI) broth (BD, BBLTM, catalog number: 211060 )
- DifcoTM brain heart infusion (BHI) agar (BD, DifcoTM, catalog number: 241810 )
- Chloramphenicol (Alfa Aesar, catalog number: B20841 )
- 100% ethanol (Decon Labs, catalog number: V1016TP )
- Neomycin sulfate (AMRESCO, catalog number: 0558-25G )
- Magnesium chloride (MgCl2) (AMRESCO, catalog number: J364 )
- Tris (AMRESCO, catalog number: 0497 )
- Hydrochloric acid (HCl) (VWR, BDH®, catalog number: BDH3030-2.5LPC )
- Potassium chloride (KCl) (VWR, BDH®, catalog number: BDH9258-500G )
- EDTA, disodium salt, dihydrate (EMD Millipore, OmniPur®, catalog number: 4050 )
- Sodium hydroxide (NaOH) (AMRESCO, catalog number: 0583 )
- Sodium dodecyl sulfate (SDS) (VWR, catalog number: 97064-862 )
- Bromophenol blue (VWR, catalog number: 97061-690 )
- Ambicin® L (Recombinant lysostaphin) (AMBI, catalog number: LSPN-50 )
- Sodium acetate anhydrous (NaOAc) (AMRESCO, catalog number: 0602 )
- Sodium phosphate monobasic (NaH2PO4) (AMRESCO, catalog number: 0571 )
- Sodium chloride (NaCl) (VWR, BDH®, catalog number: BDH9286 )
- Glycerol (AMRESCO, catalog number: M152 )
- Beta-mercaptoethanol (β-ME) (Geno Technology, G-Biosciences, catalog number: BC98 )
- Coomassie Blue G-250 (AMRESCO, catalog number: M140-10G )
- Methanol (VWR, BDH®, catalog number: BDH20864.400 )
- Acetic acid (HAc) (VWR, BDH®, catalog number: BDH3096-2.5LPC )
- Tris-glycine-SDS 10x buffer (AMRESCO, catalog number: 0783-5L )
- Imidazole (Alfa Aesar, catalog number: A10221 )
- Pierce protease and phosphatase inhibitor mini tablets, EDTA-free (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88669 )
- Triton X-100 surfactant (EMD Millipore, catalog number: TX1568 )
- Potassium hydroxide (KOH) (Alfa Aesar, catalog number: 13451 )
- Media and antibiotics (see Recipes)
- Brain heart infusion (BHI) broth
- Chloramphenicol stock (10 mg/ml)
- Neomycin stock (15 mg/ml)
- Stock solutions (see Recipes)
- 1 M MgCl2
- 1 M Tris-HCl, pH 8.3
- 1 M Tris-HCl, pH 6.8
- 1 M KCl
- 500 mM EDTA, pH 8.0
- 10% sodium dodecyl sulfate (SDS)
- 1% bromophenol blue
- 2 mg/ml recombinant lysostaphin
- 2x lysis buffer
- 5x annealing buffer
- 5x protein loading buffer
- Coomassie Blue staining solution
- Destaining solution
- 1x Tris-glycine buffer
- Buffers to prepare on Day 4 for purification–Part 1 (see Recipes)
- Elution buffer
- Resuspension buffer
- Equilibration buffer
- Wash 1 buffer
- Wash 2 buffer
Equipment
- Eppendorf Research® plus pipettes set (Eppendorf, catalog number: 2231000222 ), or equivalent pipettes set with a range of 1 μl to 1,000 μl
- Media/storage bottles (250 ml) (VWR, catalog number: 10754-816 ), or equivalent
- Erlenmeyer flask (2 L) (VWR, catalog number: 10545-844 ), or equivalent
- Standard orbital shaker (VWR, model: 1000, catalog number: 89032-088 ), or equivalent shaker that can gently rotate for gel staining and destaining
- New Brunswick I26 incubating shakers (Eppendorf, New BrunswickTM, model: I26 , catalog number: M1324-0008), or equivalent shaking incubator that can maintain 37 °C and 180 rpm
- Heraeus Multifuge X1R centrifuge series (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM X1R , catalog number: 75004251), or equivalent refrigerated centrifuge with a rotor capable of holding 400 ml bottles and applying 13,000 x g of centrifugal force
- General purpose water baths (VWR, model: VWR General Purpose Water Baths, catalog number: 89501-460 ), or equivalent capable of maintaining 37 °C
- Sonifier® S-450 Analog sonicator (Emerson, Branson, model: S-450 , catalog number: 101-063-198), or equivalent ultrasonic homogenizer with operating frequency of 20 kHz and tip diameter of 3.2 mm
- 30 ml beaker (Corning, PYREX®, catalog number: 1000-30 ) or equivalent
- Digital dry block heaters (VWR, catalog number: 12621-088 ), or equivalent block heater capable of heating up to 95 °C
- Magnetic bead separation rack (Thermo Scientific, catalog number: MR02 ), or equivalent rack capable of collecting magnetic beads
- Autoclave (Getinge), or equivalent autoclaving instrument capable of heating up to 121 °C while applying 15 atm pressure
- Graduated cylinder (100 ml) (VWR, catalog number: 65000-006 ), or equivalent
- Graduated cylinder (1 L) (VWR, catalog number: 65000-012 ), or equivalent
- Media/storage bottles (1 L) (VWR, catalog number: 10754-820 ), or equivalent
- Media/storage bottles (100 ml) (VWR, catalog number: 10754-814 ), or equivalent
- Media/storage bottles (500 ml) (VWR, catalog number: 10754-818 ), or equivalent
- UltrospecTM 10 cell density meter (GE Healthcare, catalog number: 80-2116-30 ), or equivalent spectrophotometer that can measure the density of cells in suspension at 600 nm
- Mini-PROTEAN tetra cell (Bio-Rad Laboratories, model: Mini-PROTEAN Tetra Cell, catalog number: 1658004EDU ), or equivalent vertical electrophoresis system
- pH meter AccumetTM AB15 plus basic (Fisher Scientific, model: Fisher ScientificTM accumetTM AB15+ Basic and BioBasicTM, catalog number: 13-636-AB15P ), or equivalent pH meter with a range of 0.000-10.000
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Chou-Zheng, L. and Hatoum-Aslan, A. (2017). Expression and Purification of the Cas10-Csm Complex from Staphylococci. Bio-protocol 7(11): e2353. DOI: 10.21769/BioProtoc.2353.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 分离和纯化
分子生物学 > 蛋白质 > 表达
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link