- EN - English
- CN - 中文
Reversible Cryo-arrests of Living Cells to Pause Molecular Movements for High-resolution Imaging
采用活细胞可逆性冷冻停滞法暂停分子运动以进行高分辨率成像
(*contributed equally to this work) 发布: 2017年04月20日第7卷第8期 DOI: 10.21769/BioProtoc.2236 浏览次数: 8582
评审: Vivien Jane Coulson-ThomasFederica PisanoAnonymous reviewer(s)
Abstract
Fluorescence live-cell imaging by single molecule localization microscopy (SMLM) or fluorescence lifetime imaging microscopy (FLIM) in principle allows for the spatio-temporal observation of molecular patterns in individual, living cells. However, the dynamics of molecules within cells hamper their precise observation. We present here a detailed protocol for consecutive cycles of reversible cryo-arrest of living cells on a microscope that allows for a precise determination of the evolution of molecular patterns within individual living cells. The usefulness of this approach has been demonstrated by observing ligand-induced clustering of receptor tyrosine kinases as well as their activity patterns by SMLM and FLIM (Masip et al., 2016).
Keywords: Cryo-arrest (冷冻停滞)Background
Understanding molecular processes in cells, e.g., the ligand-induced response of receptor-tyrosine kinases (RTKs), requires the precise spatio-temporal observation of molecular patterns. Due to variance in cellular states, this response needs to be monitored in individual cells rather than in cell populations (Snijder and Pelkmans, 2011). Using SMLM, individual molecules can be localized with high precision (Betzig et al., 2006). This allows, for instance, extracting information about the clustering of RTKs in the plasma membrane. Complementarily, confocal FLIM can unveil how molecules react as an ensemble within a diffraction-limited volume element. This can reveal interaction patterns of RTKs with downstream molecules, phosphorylation patterns as well as activity patterns by the use of conformational sensors (Offterdinger et al., 2004; Sabet et al., 2015). However, the acquisition time in FLIM and SMLM is in the order of minutes. Because many molecular arrangements in living cells–including those of RTKs–evolve on a much faster time scale, confocal FLIM images get blurred and spatial resolution is reduced severely (Masip et al., 2016). In SMLM, molecules are localized successively in consecutive frames. Performing these measurements on dynamic molecules in living cells will lead to a falsified image of localizations since molecules diffuse to different positions in the course of data acquisition. For instance, the epidermal growth factor receptor (EGFR) moves on average with a diffusion constant of 2-5 10-2 μm2/sec in the 2D environment of the membrane (Orr et al., 2005; Xiao et al., 2008). To allow for long acquisition times, irreversible chemical fixation with aldehydes is typically used. Yet, the use of chemical fixation agents is inefficient in immobilizing membranes and can lead to protein extraction or artificial clustering (Saffarian et al., 2007; Tanaka et al., 2010; Schnell et al., 2012). Further, the lethal fixation disrupts the out-of-equilibrium physiological state and prohibits the observation of the evolution of molecular patterns within an individual cell. We have therefore developed a reversible cryo-arrest that halts molecular movements for high-resolution imaging, yet maintains cells in a viable state. Upon cooling, the concentration of cryoprotective dimethyl sulfoxide (DMSO) is increased stepwise (Masip et al., 2016). This precludes the formation of ice crystals that can be lethal to cells while scattering light and altering cellular structure by displacing organic material (Dubochet et al., 1988 and 2012; Huebinger et al., 2016). At the same time, adding DMSO at low temperatures greatly reduces its toxicity (Farrant, 1965). A further advantage of the cryo-approach is that fluorophores become less reactive in the excited state, resulting in the emission of more photons before bleaching as well as a lowered production of cytotoxic radicals (Kaufmann et al., 2014), which can severely damage cells during acquisition at physiological temperatures (Wäldchen et al., 2015).
Materials and Reagents
- 10 μm thick double-sided adhesive tape (D80 19 x 50 mm) (Modulor, catalog number: 0332054 )
- Cover slides 21 x 26 mm (No.1) (Thermo Fisher scientific, Thermo scientificTM, catalog number: BBAD02100260#A* )
- 6-well plates for cell culture (SARSTEDT, catalog number: 83.3920 )
- 15-ml reaction tubes (e.g., SARSTEDT, catalog number: 62.554.502 )
- 200-μl pipette tips (e.g., Greiner Bio One International, catalog number: 739290 )
- Silicon tube
- Flexible silicon tubes (inner diameter [i.d.] 2 mm; outer diameter [o.d.] 4 mm and i.d. 1.5 mm; o.d. 3 mm) (e.g., VWR, catalog numbers: 228-0704 and 228-0702 )
- Cell line of interest and suitable cell culture medium
Note: The protocol has so far been tested for the adherently growing cell lines HeLa (ATCC, catalog number: CCL-2 ), MDCK (ATCC, catalog number: CCL-34 ), COS-7 (ATCC, catalog number: CRL-1651 ), MCF7 cells (ATCC, catalog number: HTB-22 ) and HCT116 (ATCC, catalog number: CCL-2 47). Other cell lines may be used after proper testing for the reversibility of the cryo-arrest. - Ethanol, absolute (e.g., Fisher Scientific, catalog number: 10342652 )
- Sterile water (sterilized by filtration)
- DMSO (min 99%) (e.g., Serva Electrophoresis, catalog number: 20385 )
- Liquid nitrogen
- Immersion oil (e.g., Olympus, catalog number: IMMOIL-F30CC )
- Transfection reagents and plasmid expressing fluorescent proteins of interest or cell lines stably expressing a fluorescent protein of interest. The protocol has been successfully used for confocal FLIM with a conformational activity sensor for EphrinA2 and EGFR tagged to mCitrine in combination with a phosphotyrosine binding domain tagged to mCherry. Single molecule localization has been performed with EGFR-mEos2 and with Vinculin tagged to a SNAP-Tag and labelled with SNAP-Cell TMR-star (New England Biolabs, catalog number: S9105S ). Requests about these plasmids can be addressed to the corresponding author
- HEPES or phosphate buffered imaging medium without phenol red (e.g., DMEM, PAN-Biotech, catalog number: P04-01163 )
- 10% fetal bovine serum (FBS) (PAN-Biotech, catalog number: P30-1505 )
- Streptomycin/penicillin (PAN-Biotech, catalog number: P06-07100 )
- L-glutamine (200 mM) (PAN-Biotech, catalog number: P04-80100 )
- 1% nonessential amino acids (PAN-Biotech, catalog number: P08-32100 )
- Different DMSO solutions (see Recipes)
- Cell culture medium (see Recipes)
Equipment
- Scalpel
- Laminar flow hood
- Incubator (e.g., Nuaire, model: NU-5510/E )
- Fine forceps (e.g., Electron Microscopy Sciences, catalog number: 0203-7-PO )
- Anodized (black) aluminum flow-through chamber (custom-built; see Figure 1)
Note: Anodizing the aluminum reduces its corrosion and thereby solvation of aluminum ions, which might otherwise influence cellular reactions. Anodizing with black color reduces reflection of light, which might interfere with the microscopic imaging.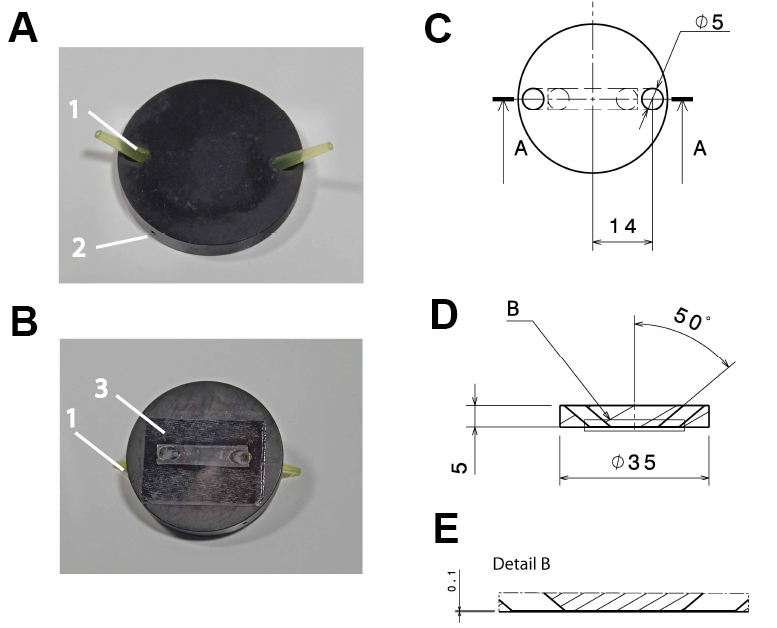
Figure 1. Design of the flow-through chamber. Photographic representation (A, B) and technical drawings (C-E) of the self-built flow-through chamber made out of aluminum. A. Top view on the flow through chamber with pipette tips inserted into the in- and outlet (1) and a drill hole (2; depth: 15 mm; diameter: 1 mm) for the insertion of a thermocouple. B. Bottom view with the cover slide glued to the flow-through chamber (3); C. Top view of the flow-through chamber; D. Side view section through the flow-through chamber (section a:a in C); E. Detail of the side view section (detail b in D). All dimensions in c-e are in mm. - Polyvinyl chloride (PVC) insert for the microscope table (custom-built; see Figure 2), with 2 threaded holes to fix the stage using a metal clamp (see Figure 3B)
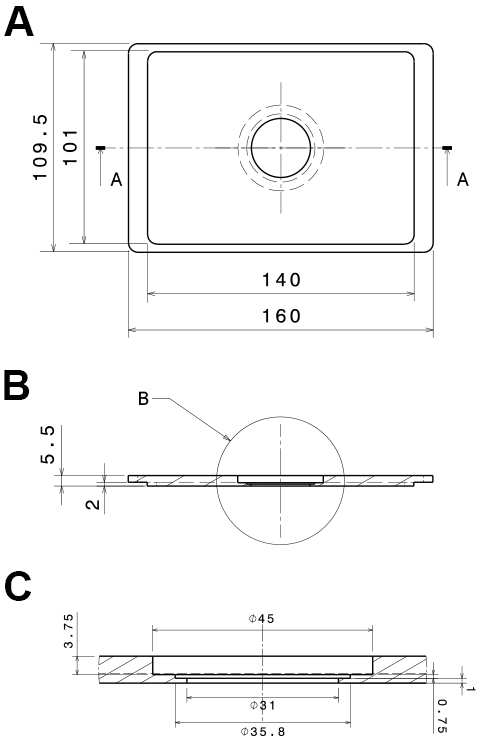
Figure 2. Technical, drawings of the PVC-insert for the microscope stage. The technical drawings show a mounting that fits into Scan IM stages (Märzhäuser Wetzlar GmbH & Co. KG, Wetzlar, Germany). A. Bottom view; B. Side view section (a:a in A); C. Side view detail of the central part (b in B). All dimensions are in mm. - Low-pressure syringe pump with a computer interface (Cetoni, model: neMESYS low pressure syringe pump )
- Cryo-stage with temperature control (Linkam Scientific, model: MDS600 , other models with similar cooling heads may also work)
- Thermocouple (e.g., PTFE-insulated type T with 0.08 mm wires; Omega Engineering, Deckenpfronn, Germany) with a data acquisition device connected to a computer for continuous temperature recording (e.g., OMEGA Engineering, model: OMB-DAQ-2408-2AO )
- For confocal FLIM: Confocal laser scanning microscope equipped with a time correlated single photon counting unit and a ps-pulsed laser (e.g., PicoQuant, model: MicroTime 200 )
- For SMLM: Microscope equipped with sensitive cameras (EMCCD or sCMOS) as well as lasers with adequate wavelength and power to image, switch on and bleach fluorophores
- Inverted microscope (Olympus, model: IX-81 )
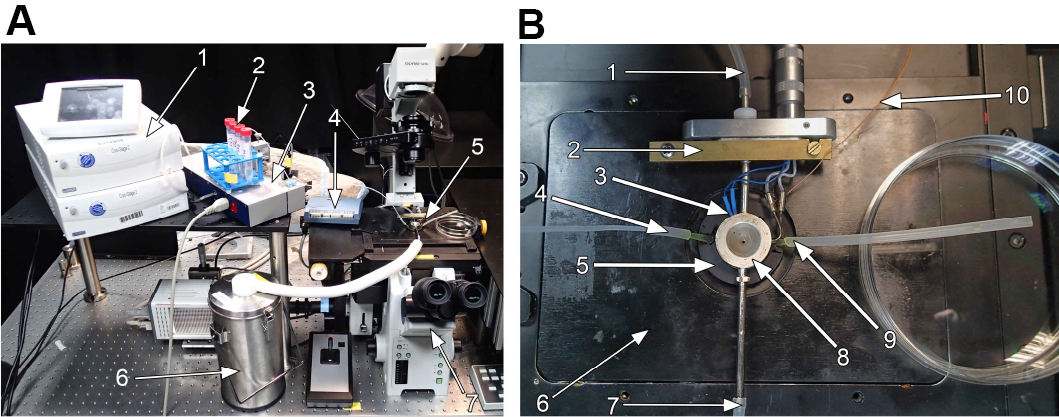
Figure 3. Cryo-stage mounted on a microscope. Photographic representation of the cryo-stage mounted on an inverted microscope ( IX-81 ; Olympus GmbH, Hamburg, Germany). A. Overview of the cryo-stage with peripheral instruments on the microscope. 1: Liquid nitrogen pump with control unit (Note: This should normally be disconnected from the optical table, since it may cause vibrations of the sample); 2: 15-ml tubes with medium of different DMSO-concentrations, inlet tube of the low-pressure syringe pump is inserted via a lid with a hole; 3: Low-pressure syringe pump; 4: Data acquisition device for thermocouple; 5: Central part of the cryo-stage as detailed in (B); 6: Liquid nitrogen reservoir. B. Detailed image of the central part of the microscope. 1: Tube connected to nitrogen pump; 2: Metal clamp to fix the stage to the PVC-insert; 3: Thermocouple to measure the temperature inside the silver block; 4: Medium inlet connected to the low pressure syringe pump; 5: Aluminum flow-through chamber (compare Figure 1); 6: PVC-insert for microscope stage (compare Figure 2); 7: Tube connected to the nitrogen reservoir; 8: Temperature controlled silver block with electrical counter heater; 9: Medium outlet tube; 10: Thermocouple to measure temperature of the aluminum block.
Software
- ThunderSTORM Plugin (Ovesny, Bioinformatics, 2014)
- ImageJ (Rasband, ImageJ, National Institutes of Health, Bethesda, Maryland, USA)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Huebinger, J., Masip, M. E., Christmann, J., Wehner, F. and Bastiaens, P. I. H. (2017). Reversible Cryo-arrests of Living Cells to Pause Molecular Movements for High-resolution Imaging. Bio-protocol 7(8): e2236. DOI: 10.21769/BioProtoc.2236.
分类
细胞生物学 > 细胞成像 > 活细胞成像
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link