- EN - English
- CN - 中文
Time-lapse Observation of Chromosomes, Cytoskeletons and Cell Organelles during Male Meiotic Divisions in Drosophila
果蝇雄性减数分裂期间染色体、细胞骨架和细胞器的延时观察
发布: 2017年04月20日第7卷第8期 DOI: 10.21769/BioProtoc.2225 浏览次数: 10979
评审: Jihyun KimFilipa VazAnonymous reviewer(s)
Abstract
In this protocol, we provide an experimental procedure that perform time-lapse observation of intra-cellular structures such as chromosomes, cytoskeletons and cell organelles during meiotic cell divisions in Drosophila males. As primary spermatocyte is the largest dividing diploid cell in Drosophila, which is equivalent in size to mammalian cultured cells, one can observe dynamics of cellular components during division of the model cells more precisely. Using this protocol, we have showed that a microtubule-associated protein plays an essential role in microtubule dynamics and initiation of cleavage furrowing through interaction between microtubules and actomyosin filaments. We have also reported that nuclear membrane components are required for a formation and/or maintenance of the spindle envelope essential for cytokinesis in the Drosophila cells.
Keywords: Drosophila (果蝇)Background
In Drosophila, good cultured cell lines that proliferate well in a standard culture condition are also available. However, their cell size, particularly cytoplasmic volume, is much smaller than that of mammalian cells. This compromises the examination of cellular components during cell division. Spermatocytes, on the other hand, achieve distinct cell growth before initiation of first meiotic division. The primary spermatocytes are the largest diploid cells among proliferative cells to appear in Drosophila development. Thus, one can easily perform detailed observation of cellular structures in dividing cells using optical microscopes. In Drosophila melanogaster, well-advanced and sophisticated genetic techniques are available (Ashburner et al., 2004). Meiotic defects in chromosome segregation and in cytokinesis appear in cellular organization of spermatids just after completion of 2nd meiotic division. By observation of such early spermatids, one can easily find out even subtle meiotic abnormalities (Giansanti et al., 2012; Inoue et al., 2012). Furthermore, if a loss of microtubule integrity or dynamics would have occurred in normal cultured cells, their cell cycle progression should be arrested before metaphase. Therefore, it is hard to examine how microtubules would influence later processes of cell divisions in the somatic cells. Spermatocytes, on the other hand, are less sensitive to microtubule abnormalities at microtubule assembly checkpoint before metaphase. One can, therefore, examine a role of microtubule-related genes in cytokinesis without arresting cell cycle. We and other groups have established systems to facilitate dynamics of chromosomes or microtubules by expression of proteins fused with GFP fluorescence tag (Clarkson and Saint, 1999; Inoue et al., 2004; Rebollo and Gonzalez, 2004; Kitazawa et al., 2012).
Previous protocols can trap the male meiotic cells in a narrow space sandwiched between a coverslip and a slide glass, ensured by a small cushion materials and observe chromosome segregation under an upright microscope (Savoian et al., 2000; Inoue et al., 2004; Savoian, 2015). These protocols allowed us to collect clear images of microtubules. However, a preparation that makes the cells flattened often prevents initiation and/or progression of cytokinesis. In addition, it was difficult to add drugs or inhibitors to the living cells while time-lapse observation.
Therefore, we have established a new method that allows us to observe a whole meiosis I from prophase I to end of cytokinesis in an open chamber under an inverted microscope. We can add drugs in the cell culture in any timing of the imaging. We also improved the protocol so that we can perform a simultaneous observation of chromosomes and other cellular components such as microtubules, actin filaments, endoplasmic reticulum, Golgi apparatuses or mitochondria during male meiosis I. It can be achieved by a simultaneous expression of proteins fused with different fluorescent tags showing spectrally separable colors. As most aspects of division process seen in Drosophila meiotic cells are shared among higher eukaryotes, this protocol should be useful for studying cell division processes of other organisms as well as Drosophila somatic cell mitosis.
Materials and Reagents
- Cover slips (22 x 22 mm, No. 1, thickness 0.12-0.17 mm) (Matsunami Glass, catalog number: C022221 )
Note: It was argued that thorough cleaning steps of cover slips are required for maintenance of cell viability and for a success prolonged observation of living cells (Savoian, 2015). However, if these cover slips are used, any pretreatment is not basically necessary except a wipe with 70% ethanol just before using. - Plastic cover slip folder (76 x 26 mm)
Note: The folders were customized. It was 76 x 32 mm in length and width and 1.7 mm in thickness. There was a concavity (25 mm square) where the cover slip fell in the surface and a circular hole of 15 mm diameter in the center of the concavity) (see Figure 1).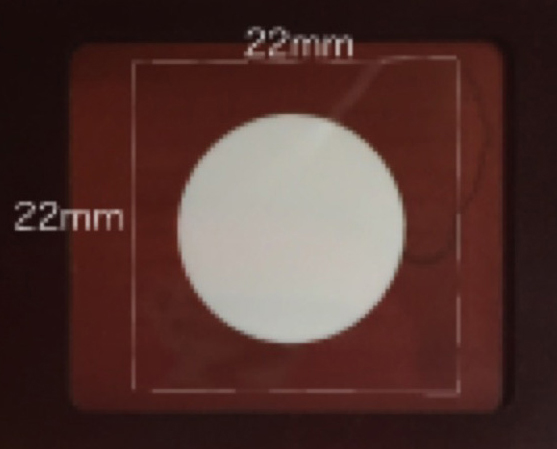
Figure 1. A plastic cover slip holder. The holder should be set on the microscope stage. There was a concavity where the cover slip (22 x 22 mm) fell in the surface and a circular hole with 15 mm diameter in the center of the concavity. - 10 x 10 mm open frame that had adhesives on the bottom side (Gene Frame 25 μm) (Thermo Fisher Scientific Co., Waltham, USA)
- Plastic Petri dish (90 mm diameter) (AsOne, catalog number: 1-7484-01 )
- Kim-wipe (KCWW, Kimberly-Clark)
- bam-Gal4::vp16 (abbreviated as bam-Gal4) can be used as a Gal4 driver for testis-specific ectopic expression of fluorescence proteins (Kitazawa et al., 2012)
- bam-Gal4::vp16 UAS-dir2 was used as a Gal4 driver for testis-specific depletion (Kitazawa et al., 2014)
- P{His2AvT:Avic\GFP-S65T} (abbreviated as Histone2Av-GFP) can be used for expression of Histone 2Av fused with a GFP tag to visualize chromatin in living meiotic cells (Bloomington Drosophila Stock Center, catalog number: BL5941 )
- P{Ubi-mRFP-βTub85D} (abbreviated as RFP-βTubulin) can be used for ubiquitous expression of β-tubulin fused with a mRFP tag to visualize microtubules in living meiotic cells (Kitazawa et al., 2014)
- P{sqh-EYFP-Golgi} can be used for ubiquitous expression of Golgi components fused with a YFP tag to visualize Golgi apparatus in living meiotic cells (Bloomington Drosophila Stock Center, catalog number: BL7193 )
- P{sqh-EYFP-ER} can be used for ubiquitous expression of ER components fused with a YFP tag to visualize endoplasmic reticulum in living meiotic cells (Bloomington Drosophila Stock Center, catalog number: BL7195 )
- P{sqh-EYFP-Mito} can be used for ubiquitous expression of mitochondrial target sequences fused with a YFP tag to visualize mitochondria in living meiotic cells (Bloomington Drosophila Stock Center, catalog number: BL7194 )
- P{UASp-GFP-Orbit}, P{UASp-mRFP-Orbit}, and P{UASp-Venus-Orbit} can be used for visualization of Orbit protein, an essential microtubule-associated protein. UAS-stocks that can induce Orbit proteins fused with three different fluorescent tags are available (Miyauchi et al., 2013)
- P{UASp-mRFP-Actin5C} (Bloomington Drosophila Stock Center, catalog number: BL24777 ), P{UAS-GFP-Actin5C} (Bloomington Drosophila Stock Center, catalog number: BL9257 ) and P{UAS-CFP-Actin5C} (Miyauchi et al., 2013) can be used for ectopic expression of F-actin components fused with different tags to visualize F-actin in living meiotic cells
- P{UAS-GFP-anillin} (Bloomington Drosophila Stock Center, catalog number: BL51348 ) and P{Ub-mRFP-anillin} (Bloomington Drosophila Stock Center, catalog number: BL52220 ) can be used for visualization of a contractile ring in a living meiotic cell (gifts from J.A. Brill, now available from Bloomington Drosophila Stock Center)
- P{w[+mC]=sqh-GFP.RLC}3 can be used for ubiquitous expression of myosin light chain components fused with GFP tag to visualize MLC in living meiotic cells (a gift from R. Karess)
- P{PTT-GA}Pdi[G00198], a protein trap stock expressing GFP-Protein disulfide isomerase for visualization of intracellular membranous structures (a gift from L. Cooley, now available from Bloomington Drosophila Stock Center as catalog number: BL6839 ). A protein stock expressing GFP-LamC for visualization of nuclear lamina (a gift from L. Wallrath)
- P{UAS-PLCg-PH-GFP} for visualization of plasma membrane in male meiotic cells (a gift from J. A. Brill)
- P{UAS-mRFP-Nup107.K} 7.1 for visualization of nuclear pore complex in nuclear envelope of male meiotic cells (a gift from V. Doyle, now available from Bloomington Drosophila Stock Center as catalog number: BL35516 )
- P{UAS-GFP-Pav} and P{UAS-GFP-Polo} were used for visualization of a microtubule motor and an important cell division regulator in a living meiotic cell, respectively (gifts from D. Glover)
- For RNAi experiments in male meiotic cells, UAS-RNAi stocks for ectopic expression of dsRNA for each protein were obtained from VDRC stock center and Bloomington stock center. P{UAS-GFP RNAi} (Bloomington Drosophila Stock Center, catalog number: BL9330 ) can be used as a negative control of RNAi experiments
- Colchicine (50 μg/ml in BRB80 buffer) (≥ 95% colchicine) (Sigma-Aldrich, catalog number: C9754 )
Note: To examine requirement of microtubules for cellular dynamics, colchicine that is an inhibitor of microtubule polymerization was used. The BRB80 buffer containing colchicine was prepared before the dissection every time. The testes were dissected in the buffer containing colchicine and then, meiotic cells were spread under mineral oil. - Cytochalasin D (10 μg/ml in BRB80 buffer) (≥ 98% cytochalasin D) (Sigma-Aldrich, catalog number: C8273 )
Note: To examine requirement of F-actin for cellular dynamics, cytochalasin D that is an inhibitor of actin polymerization was used. The BRB80 buffer containing cytochalasin D was prepared before the dissection every time. The male flies were dissected to collect the testes in the buffer containing cytochalasin D and then, meiotic cells were spread under mineral oil. - Fetal calf serum (Thermo Fisher Scientific, GibcoTM, catalog number: 451456 or 10437 )
Note: The fetal calf serum can be kept at 4 °C for a month. - Mineral oil (Trinity Biotech, catalog number: 400-5-1000 )
Note: The mineral oil was replaced to a fresh one every time-lapse recording. - Brefeldin A (Cell Signaling, catalog number: 9972 ) or Exo1 (Sigma-Aldrich, catalog number: E8280 )
Note: To examine whether membrane trafficking mediated by COPI is required for cellular dynamics, each compound was used to inhibit αCOPI. They were directly added to the culture medium. The BRB80 buffer containing Brefeldin A or Exo1 was prepared before the dissection every time. The testis can be incubated in the culture medium for up to 14 h before isolation of spermatocytes. - PIPES (Dojindo Mecular Technologies, catalog number: 340-08255 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Nacalai Tesque, catalog number: 20908-65 )
- Ethylene Glycol Bis (EGTA) (Nacalai Tesque, catalog number: 08907-42 )
- Potassium chloride (KCl) (Wako Pure Chemical Industries, catalog number: 162-17942 )
- Sodium chloride (NaCl) (Wako Pure Chemical Industries, catalog number: 191-01665 )
- Sodium phosphate dibasic (Na2HPO4)
- Potassium dihydrogen phosphate (KH2PO4)
- M3 medium (Sigma-Aldrich, catalog number: S8398 )
- BRB80 buffer (pH 6.8) (see Recipes)
- Insect M3 medium (see Recipes)
- Phosphate-buffered saline (PBS) (see Recipes)
Equipment
- Super fine forceps (Fine Science Tools, model: Dumont #5 )
- Dissection needles sharpened tungsten wire with 0.5 mm diameter
- Inverted fluorescence microscope (Olympus, model: IX81 ) outfitted with excitation, emission filter wheels (Olympus, Tokyo, Japan)
- Objectives; UPLFLN40XPH (NA=0.75), UPLSAPO60XO (NA=1.4), UPLSAPO100XO (NA=1.4) (Olympus, Tokyo, Japan)
- Hg lump (Olympus, catalog number: USH-1030L )
- Cooled CCD camera (Hamamatsu Photonics, model: C10600-10B )
- Autoclave
Software
- Metamorph software version 7.6 (Molecular Devices, Sunnyvale, USA)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Tanabe, K., Okazaki, R., Kaizuka, K. and Inoue, Y. H. (2017). Time-lapse Observation of Chromosomes, Cytoskeletons and Cell Organelles during Male Meiotic Divisions in Drosophila. Bio-protocol 7(8): e2225. DOI: 10.21769/BioProtoc.2225.
分类
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













