- EN - English
- CN - 中文
Production, Purification and Crystallization of a Prokaryotic SLC26 Homolog for Structural Studies
生成、纯化和结晶原核生物中SLC26同源蛋白用于结构研究
发布: 2017年02月05日第7卷第3期 DOI: 10.21769/BioProtoc.2116 浏览次数: 9551
评审: Arsalan DaudiChing Yao YangAnonymous reviewer(s)

相关实验方案
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 1284 阅读
Abstract
The SLC26 or SulP proteins constitute a large family of anion transporters that are ubiquitously expressed in pro- and eukaryotes. In human, SLC26 proteins perform important roles in ion homeostasis and malfunctioning of selected members is associated with diseases. This protocol details the production and crystallization of a prokaryotic SLC26 homolog, termed SLC26Dg, from Deinococcus geothermalis. Following these instructions we obtained well-folded and homogenous material of the membrane protein SLC26Dg and the nanobody Nb5776 that enabled us to crystallize the complex and determine its structure (Geertsma et al., 2015). The procedure may be adapted to purify and crystallize other membrane protein complexes.
Keywords: Membrane transport protein (膜转运蛋白)Background
With few exceptions, structural characterization of membrane proteins involves challenges at the level of protein production, stabilization in the detergent-solubilized state, and crystallization. The strategy we have followed to overcome these hurdles relied on the efficient selection of SLC26 homologs with superior biochemical properties and the use of antibodies as crystallization chaperones (Geertsma et al., 2015). The procedures described here do not greatly deviate from those of colleagues, but on a few points we do follow alternative approaches. For example, for protein production we make use of the araBAD promoter (Guzman et al., 1995) and not the popular T7 promoter (Studier et al., 1990). In contrast to the T7 promoter, the PBAD promoter allows direct tuning of the protein production levels and its adjustment to the capacity of the downstream folding machinery, thereby reducing the formation of inclusion bodies (Geertsma et al., 2008). Furthermore, we prefer nanobodies, the variable domain of camelid heavy chain only antibodies (Pardon et al., 2014), as crystallization chaperones over the more commonly used Fabs. In our hands, the generation, selection, and production of nanobodies is far more robust and straightforward. Though we are aware that alternative protein production strategies (Henderson et al., 2000; Kunji et al., 2003; Miroux and Walker, 1996; Studier, 2005; Wagner et al., 2008) and crystallization chaperones (Koide, 2009; Seeger et al., 2013) exist, we did not explore these as the presented procedures proved very robust and successful.
Materials and Reagents
- Dialysis tube
MWCO 8 kDa (Carl Roth, catalog number: 1924.1 )
MWCO 3.5 kDa (Carl Roth, catalog number: E860.1 ) - Concentrators
MWCO 50 kDa (EMD Millipore, catalog number: UFC905024 )
MWCO 3 kDa (EMD Millipore, catalog number: UFC800324 ) - Crystallization plates (HAMPTON RESEARCH, catalog number: HR3-158 )
- Potter tube and piston (VWR, catalog numbers: 432-0205 and 432-0211 )
- 50 ml tube
- E. coli MC1061 (Coli Genetic Stock Center, catalog number: 6649 )
- Plasmid pBXNPHM3-Nb5776 (Figure 1A)
Note: This plasmid holds the gene coding for the nanobody under control of the araBAD promoter and results in the production of the nanobody fused to an N-terminal pelB leader sequence followed by decaHis-tag, MBP, and a HRV 3C protease site. It contains a beta-lactam antibiotic marker. Plasmid available for non-commercial use upon request. - Plasmid pBXC3GH-SLC26Dg (Figure 1B)
Note: This plasmid holds the gene coding for SLC26Dg under control of the araBAD promoter and results in the production of SLC26Dg fused to a C-terminal HRV 3C protease site, GFP, and a decaHis-tag. It contains a beta-lactam antibiotic marker. Plasmid available for non-commercial use upon request.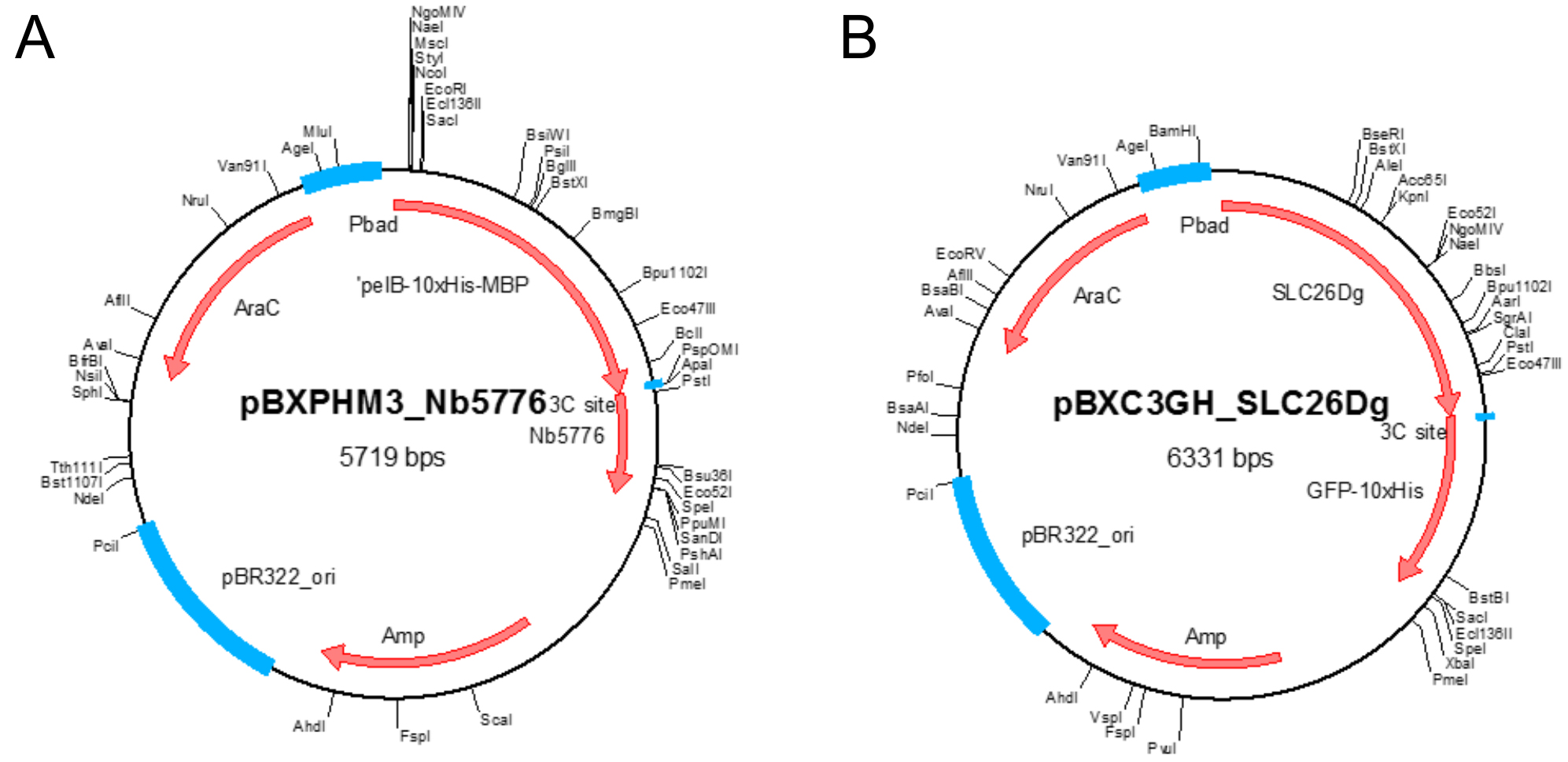
Figure 1. Plasmid maps. A. Plasmid map of pBXNPHM3-Nb5776; B. Plasmid map of pBXC3GH-SLC26Dg. Unique restriction sites and important features in the plasmids are indicated. - Ampicillin sodium salt (Carl Roth, catalog number: K029.2 )
- L-arabinose (20% w/v in ddH2O) (Carl Roth, catalog number: 5118.2 )
- 1 M KPi, pH 7.5 (see Recipes)
K2HPO4(AppliChem, catalog number: 121512 )
KH2PO4(AppliChem, catalog number: 131509 ) - Sodium chloride (NaCl, 2.5 M, ddH2O) (Carl Roth, catalog number: 3957.1 )
- Lysozyme (100 mg/ml in ddH2O) (AppliChem, catalog number: A3711 )
- DNAse I (2 mg/ml in ddH2O) (AppliChem, catalog number: A3778 )
- Magnesium sulphate heptahydrate (MgSO4, 1 M, ddH2O) (Carl Roth, catalog number: P027.2 )
- Phenylmethyl sulphonyl fluoride (PMSF, 200 mM in ethanol) (Carl Roth, catalog number: 6367.1 )
- NiNTA (50% w/v slurry in 20% EtOH) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88223 )
- ddH2O
- Imidazole (2 M, pH 7.5, HCl, ddH2O) (Carl Roth, catalog number: X998.4 )
- HEPES (1 M, pH 7.5, NaOH, ddH2O) (Carl Roth, catalog number: HN78.2 )
- HRV 3C protease (homemade)
- Polypropylene glycol 2000 (10% v/v in ddH2O) (Sigma-Aldrich, catalog number: 81380-1L )
- EDTA, disodium salt dihydrate (Carl Roth, catalog number: X986.1 )
- Glycerol, 86% (w/v) (Carl Roth, catalog number: 4043.3 )
- Liquid nitrogen
- Decylmaltoside (10% w/v in ddH2O) (Anatrace, catalog numbers: D322S and D322LA )
- Ammonium formate (Carl Roth, catalog number: 5093.1 )
- Sodium acetate (Na-acetate) (Carl Roth, catalog number: 6773.2 )
- PEG400 (Sigma-Aldrich, catalog number: 91893 )
- Tryptone (AppliChem, catalog number: A1553 )
- Yeast extract (AppliChem, catalog number: A1552 )
- Agar (Applichem, catalog number: A0917 )
- LB agar (see Recipes)
- TB medium (see Recipes)
- Reservoir solution (see Recipes)
Equipment
- 5 L baffled flask
- Incubator at 37 °C
- Shaker with adjustable temperature (Infors, model: HT Multitron )
- Centrifuge for pelleting cultures (Thermo Fisher Scientific, Thermo Scientific, model: Thermo Sorvall Evolution RC )
- Homogenizer (IKA, model: ULTRA-TURRAX® T25 )
- High pressure cell disrupter (Avestin, model: Emulsiflex C3 )
- Nanodrop (Thermo Fisher Scientific, NanodropTM, model: 1000 , discontinued)
- Magnet stirrer (Heidolph Instruments, model: Hei-Mix S )
- SEC column Superdex 75 10/300 GL (GE Healthcare, catalog number: 17-5174-01 )
- SEC column Superdex 200 10/300 GL (GE Healthcare, catalog number: 28-9909-44 )
- Table-centrifuge (VWR, model: Micro Star 17 )
- HPLC (GE Healthcare, model: ÄKTAprime plus )
- Fermenter (BIOENGiNEERiNG, model: NLF 22, 30 L)
- Spectrophotometer (Amersham Biosciences, model: Ultrospec 10 )
Note: This product has been discontinued. - Ultracentrifuge (Beckman Coulter, model: Optima XPN-100K Ultracentrifuge )
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Chang, Y., Shaik, F. R., Neldner, Y. and Geertsma, E. R. (2017). Production, Purification and Crystallization of a Prokaryotic SLC26 Homolog for Structural Studies. Bio-protocol 7(3): e2116. DOI: 10.21769/BioProtoc.2116.
分类
微生物学 > 微生物生物化学 > 蛋白质
生物化学 > 蛋白质 > 表达
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link