- EN - English
- CN - 中文
P-body and Stress Granule Quantification in Caenorhabditis elegans
秀丽隐杆线虫中P-body和应激颗粒的定量
发布: 2017年01月20日第7卷第2期 DOI: 10.21769/BioProtoc.2108 浏览次数: 14212
评审: Jyotiska ChaudhuriLeonardo G. GuilgurPia Giovannelli
Abstract
Eukaryotic cells contain various types of cytoplasmic, non-membrane bound ribonucleoprotein (RNP) granules that consist of non-translating mRNAs and a versatile set of associated proteins. One prominent type of RNP granules is Processing bodies (P bodies), which majorly harbors translationally inactive mRNAs and an array of proteins mediating mRNA degradation, translational repression and cellular mRNA transport (Sheth and Parker, 2003). Another type of RNP granules, the stress granules (SGs), majorly contain mRNAs associated with translation initiation factors and are formed upon stress-induced translational stalling (Kedersha et al., 2000 and 1999). Multiple evidence obtained from studies in unicellular organisms supports a model in which P bodies and SGs physically interact during cellular stress to direct mRNAs for transport, decay, temporal storage or reentry into translation (Anderson and Kedersha, 2008; Decker and Parker, 2012). The quantification, distribution and colocalization of P bodies and/or SGs are essential tools to study the composition of RNP granules and their contribution to fundamental cellular processes, such as stress response and translational regulation. In this protocol we describe a method to quantify P bodies and SGs in somatic tissues of the nematode Caenorhabditis elegans.
Keywords: Caenorhabditis elegans (秀丽隐杆线虫)Background
Thus far, most protocols to study P bodies and SGs were developed for yeast or human cell lines (Buchan et al., 2010). Little is known about the function of somatic RNP granules in multicellular organisms. The simple model organism C. elegans has been extensively used to study germline-specific P granules, which are distinct from P bodies and SGs, and important structures for germline development and function (Updike and Strome, 2010). Although the principles of the presented procedure can be applied to count germline-specific P granules, the protocol focusses on the quantification of somatic RNP granules. Several studies have identified a conserved function of somatic P bodies in the translational deregulation via miRNA pathways in C. elegans (Ding et al., 2005; Zhang et al., 2007). More recently, various tools were created to study the involvement of cytoplasmic RNP granules in cellular and organismal stress response, development and ageing in the nematode (Cornes et al., 2015; Huelgas-Morales et al., 2016; Rieckher et al., 2015; Rousakis et al., 2014; Sun et al., 2011; Table 1).
Such studies take advantage of the comparatively easy implementation of transgenesis methods in C. elegans that allow to constitutively express fluorescent fusion proteins (e.g., green fluorescent protein [GFP]), endogenously or in specific tissues (Rieckher et al., 2009). A collection of fosmids carrying gfp-tagged P body- and SG-specific genes can be obtained at the ‘C. elegans TransGeneome’ project, a genome-scale transgenic project for fluorescent- and affinity-tagged proteins for expression in the nematode (Sarov et al., 2012; Table 1). C. elegans is transparent, which allows for efficient application of fluorescence microscopy methods that are easily combined with differential interference contrast (DIC) microscopy to reveal fluorescent protein expression in an anatomical context. Mounting transgenic animals for P body and SG imaging is based on a previously described method using nanoparticles for immobilization (Kim et al., 2013), since commonly applied anesthetics in C. elegans can induce stress, resulting in increased RNP granule formation. Fluorescence-tagged P body or SG-intensity can be imaged by epifluorescence light microscopy (see Procedure C), while fluorescence intensity, a detailed count and size measurements of P bodies and SGs can be obtained via confocal laser scanning microscopy (see Procedure D).
Table 1. Tools available for transgenic expression of P body/SG-factors in C. elegans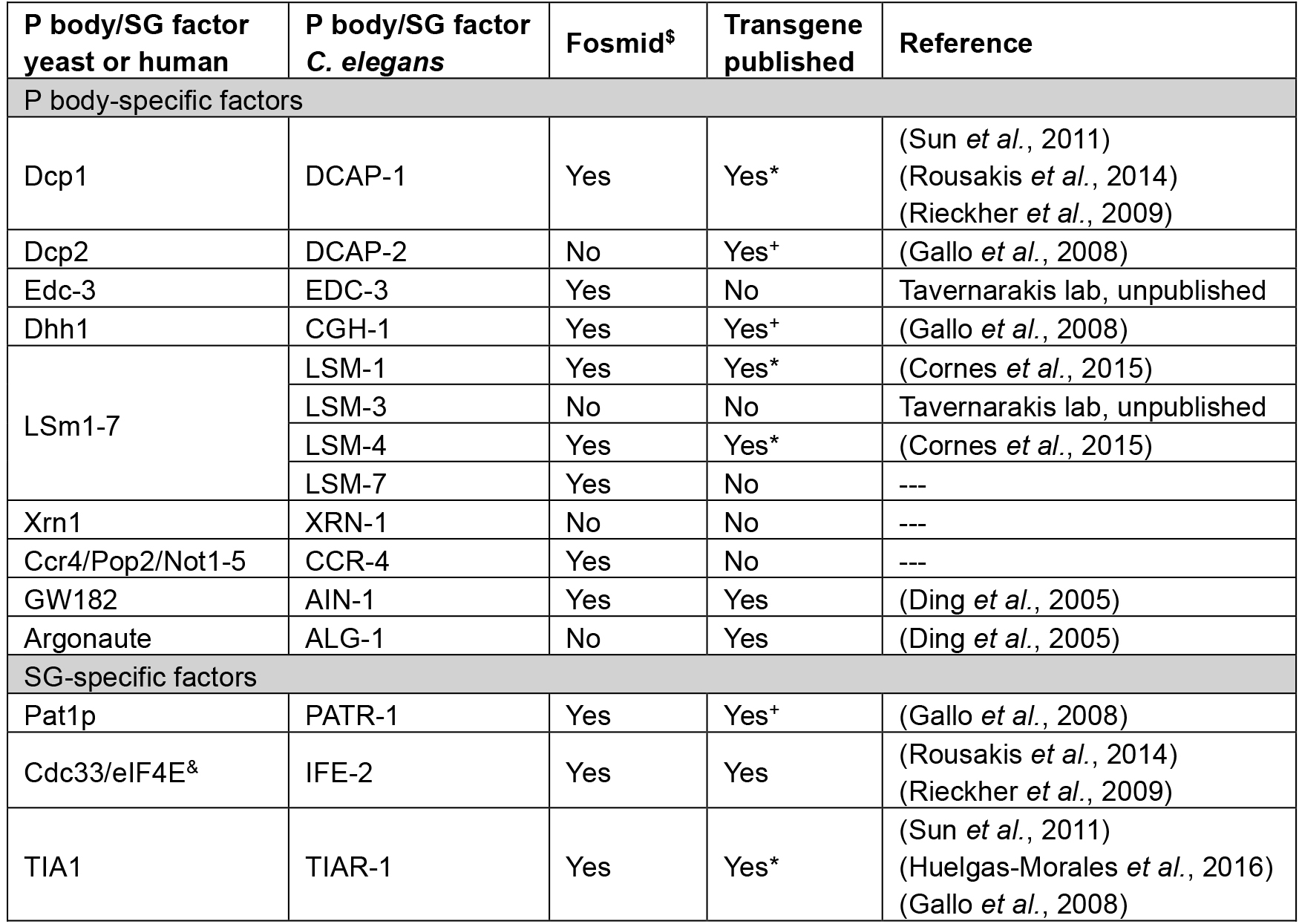
*available at CGC
+germline-specific promoter fusion
$C. elegans TransGeneome project (Sarov et al., 2012)
&can be found in both types of RNP granules
Materials and Reagents
- Sterile pipette tips
- Surgical disposable scalpel (Braun Medical, catalog number: 5518075 )
- Worm Pick with platinum wire (Genesee Scientific, catalog numbers: 59-30P6 )
- Pre-flattened tip (Genesee Scientific, catalog numbers: 59-AWP )
- Microscope slides (76 x 26 mm) (Carl Roth, catalog number: 0656.1 )
- Cover slips (24 x 24 mm) (Carl Roth, catalog number: H875.2 )
- Tape (~1 mm thickness)
- Greiner Petri dishes (60 x 15 mm) (Greiner Bio One, catalog number: 628161 )
- C. elegans strains (see Table 1 for available transgenes)
- Escherichia coli OP50 strain (obtained from the Caenorhabditis Genetics Center)
- Nail polish
- Polystyrene beads (Polybead, 2.5% by volume, 0.1 µm diameter)
- Potassium dihydrogen phosphate (KH2PO4) (Carl Roth, catalog number: P018.1 )
- Di-potassium hydrogen phosphate (K2HPO4) (Carl Roth, catalog number: 5066.1 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Di-sodium hydrogen phosphate (Na2HPO4) (Carl Roth, catalog number: T876.1 )
- Bacto peptone (BD, catalog number: 211677 )
- Streptomycin sulfate salt (Sigma-Aldrich, catalog number: S6501 )
- Agar (Sigma-Aldrich, catalog number: 05040 )
- Cholesterol stock solution (SERVA Electrophoresis, catalog number: 17101.01 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C5080 )
- Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M7506 )
- Nystatin stock solution (Sigma-Aldrich, catalog number: N3503 )
- Agarose (Biozym, catalog number: 840004 )
- Phosphate buffer (1 M; sterile) (see Recipes)
- Nematode growth medium (NGM) agar plates (see Recipes)
- M9 buffer (see Recipes)
- 5% agarose pads (see Recipes)
Equipment
- Dissecting stereomicroscope (Olympus, model: SMZ645 )
- Epifluorescence microscope (ZEISS, model: Axio Imager Z2 , objective EC Plan-Neofluar 10x/0.3)
- Confocal microscope (we use the Zeiss LSM710 confocal microscope with an Argon multiline laser source 25 mW and a tunable laser with the wavelength range 488-640 nm) (ZEISS, model: LSM710)
- Microwave
- Incubators for stable temperature (AQUA®LYTIC incubator 20 °C)
- Scale
- Cylindrical glass beaker (25 ml) (VWR, catalog number: 213-1120 )
- Autoclave
Software
- ZEN 2009 software (or later), Carl Zeiss AG, Jena, Germany (or any other software controlling a fluorescence microscope or confocal microscope)
- Microsoft Office 2011 Excel (Microsoft Corporation, Redmond, USA)
- Fiji or ImageJ (https://fiji.sc/ or https://imagej.nih.gov/ij/)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Rieckher, M. and Tavernarakis, N. (2017). P-body and Stress Granule Quantification in Caenorhabditis elegans. Bio-protocol 7(2): e2108. DOI: 10.21769/BioProtoc.2108.
- Rousakis, A., Vlanti, A., Borbolis, F., Roumelioti, F., Kapetanou, M. and Syntichaki, P. (2014). Diverse functions of mRNA metabolism factors in stress defense and aging of Caenorhabditis elegans. PLoS One 9(7): e103365.
分类
发育生物学 > 细胞信号传导 > 胁迫反应
细胞生物学 > 细胞信号传导 > 胁迫反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














