- EN - English
- CN - 中文
Allogeneic Transplantation of Testicular Hyperplasia in rag1 Mutant Zebrafish
突变斑马鱼中进行睾丸增生的同种异体移植
发布: 2016年11月05日第6卷第21期 DOI: 10.21769/BioProtoc.1992 浏览次数: 8134
评审: Raghuveer KavarthapuAnonymous reviewer(s)
Abstract
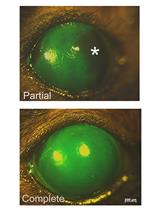
Allogeneic organ transplantation is a powerful tool for clinical and basic research studies. However, the graft is often rejected by the host organism. Here, we describe a protocol that uses immunodeficient rag1 mutant zebrafish. These zebrafish escaped rejection, which made it possible to successfully transplant fragments of an allogeneic testis and testicular hyperplasia. This protocol can be used to amplify and maintain testicular hyperplasia grafts for several years (Kawasaki et al., 2016). The amplified hyperplasias are likely to be a good source of somatic and germ cells such as Sertoli cells and spermatogonial stem cells.
Background
Zebrafish have emerged as a tractable teleost genetic model for the study of vertebrate biology because several thousand mutants have been isolated by various genetic methods (Granato and Nüsslein-Volhard, 1996). Recently, this organism was used to study human diseases such as cancer (White et al., 2013). Although the incidence of spontaneous cancers is low, with many zebrafish eventually surviving cancer, allogeneic organ transplantation is a powerful tool, because many of the cancers are not syngeneic. Unfortunately, this method is not well developed. A previous study reported that zebrafish embryos accept cell grafts prior to the development of a mature immune system (Nicoli et al., 2007). However, it is difficult to successfully transplant grafts into embryos due to their minute size. For transplantation into adult zebrafish, sublethal γ-irradiation or immunosuppression with dexamethasone can block the rejection of the graft (Stoletov et al., 2007; White et al., 2008). However, it can be difficult to maintain cell grafts for long periods of time due to the short lifespans of recipients and the recovery of the immune response by 20 days after irradiation (Smith et al., 2010; Eguiara et al., 2011). Tissue grafts between identical clonal or inbred lines can survive without rejection (Kawasaki et al., 2010; Mizgirev and Revskoy, 2010; Shinya and Sakai, 2011).
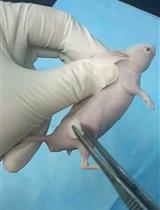
T lymphocytes are central to the allograft response (Ingulli, 2010). The Recombination activating gene 1, 2 (rag1, Rag2) are important for immune function, because it creates double-stranded DNA breaks and is essential for V(D)J recombination, as well as for T and B cell function. rag1 mutant mice lack mature T and B cells, and they maintain allogeneic heart grafts for long periods of time (Zhang et al., 2006). By contrast, allogeneic transplantation has failed in rag1 mutant rats, probably due to the insufficient depletion of T and B cells (Ménoret et al., 2013). Hypomorphic rag2E450fs mutant zebrafish has been created, which have reduced V(D)J rearrangement and lymphocytes, and maintains various allogeneic cancer cells (Tang et al., 2014). Although rag1t26683 mutant zebrafish (hereafter rag1 mutant) have been isolated and they lack functional T and B cells (Wienholds et al., 2002; Petrie-Hanson et al., 2009), they were not used for transplantation. Our recent study reported that rag1 mutant zebrafish accept and maintain allogeneic testis organ and testicular hyperplasia grafts for long periods of time (Kawasaki et al., 2016). Here, we describe a protocol that uses immunodeficient rag1 mutant zebrafish for the subcutaneous transplantation of testis and testicular hyperplasia grafts.
Materials and Reagents
- 100 mm dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 263991 )
- Paper towels
- Surgical blades (FEATHER Safety Razor, catalog number: No. 25 )
- Aluminum foil
- rag1 mutant adult male zebrafish (Wienholds et al., 2002)
- L-15 medium (Sigma-Aldrich, catalog number: L5520-500ML )
- 25x phosphate-buffered saline (PBS)
- Ethyl p-aminobenzoate (Wako Pure Chemical Industries, catalog number: 057-03832 )
- Gentamicin (10 mg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15710064 )
- Ethanol
- 10% ethyl p-aminobenzoate stock solution (see Recipes)
- 0.01% ethyl p-aminobenzoate working solution (see Recipes)
- 0.4x PBS containing 10 μg/ml gentamicin (see Recipes)
Equipment
- Stereoscopic microscope (OLYMPUS, model: SZ61 )
- Forceps (Style 5) (Dumont, catalog number: 0108-5-PO )
Procedure
文章信息
版权信息
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kawasaki, T. and Sakai, N. (2016). Allogeneic Transplantation of Testicular Hyperplasia in rag1 Mutant Zebrafish. Bio-protocol 6(21): e1992. DOI: 10.21769/BioProtoc.1992.
分类
干细胞 > 生殖细胞 > 精原干细胞
细胞生物学 > 细胞移植 > 同种异体移植
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link