- EN - English
- CN - 中文
Purification of a Protein Exhibiting Isoleucine 2-epimerase Activity from Lactobacillus otakiensis JCM 15040
乳杆菌属JCM 15040中具有异亮氨酸2-表异构酶活性的蛋白质的纯化
发布: 2015年10月20日第5卷第20期 DOI: 10.21769/BioProtoc.1632 浏览次数: 8351
评审: Timo LehtiAnonymous reviewer(s)

相关实验方案
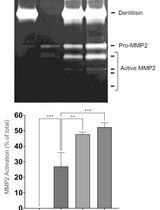
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2061 阅读
Abstract
Prominent accumulation of D-leucine, D-allo-isoleucine and D-valine was observed in the culture medium of the heterofermentative bacterial species, Lactobacillus otakiensis (L. otakiensis) JCM 15040. The racemase enzyme that resulted in this accumulation, isoleucine 2-epimerase, was purified from the bacterial cells. This is the first reported observation of such production of D-branched chain amino acids in lactic acid bacteria, and the first example of a racemase with isoleucine 2-epimerase activity in any organisms. In the described protocol, we introduce methods for purification of this protein from L. otakiensis JCM 15040. Because no specific ligand that has high affinity for this enzyme has been identified, the purification was performed using ammonium sulfate fraction, four types of column chromatography and preparative Native-PAGE, not using an affinity column chromatography. We hope that the protocol will provide useful information for purification of an enzyme that cannot easily be purified using an affinity column chromatography.
Keywords: Lactic acid bacteria (乳酸菌)Materials and Reagents
- 1.5 ml tube (ASONE Corporation, catalog number: 2-1998-02 )
- Dialysis membrane tube (molecular weight cutoff: 14,000) (EIDIA, catalog number: UC27-32-100 )
- Amicon Ultra 15 ml centrifugal filter 3 K device (Merck Millipore Corporation, catalog number: UFC500396 )
- Disposable homogenizer (1.5 ml scale) “Biomasher II” (NIPPI Corporation, catalog number: 320102 )
- Feeding tube (TERUMO CORPORATION, catalog number: SF-ET1725 )
- Syringe (10 ml scale) (TERUMO CORPORATION, catalog number: SS-10SZ )
- Plug silicon (ASONE Corporation, catalog number: 6-336-03 )
- Needle (TERUMO CORPORATION, catalog number: NN-2238R )
- Purification of the isoleucine 2-epimerase
- L. otakiensis JCM 15040 obtained from Japan Collection of Microorganisms (JCM)
- TOYOPEARL Phenyl-650M column
Note: Pack 50 ml of TOYOPEARL Phenyl-650M resin in a chromatography column (diameter: 2.5 cm, length: 10 cm) (Tosoh Bioscience LLC, catalog number: 14478 ). - TOYOPEARL Butyl-650M column
Note: Pack 50 ml of TOYOPEARL Butyl-650M resin in a chromatography column (diameter: 2.5 cm, length: 10 cm) (Tosoh Bioscience LLC, catalog number: 07477 ). - TOYOPEARL SuperQ-650M column
Note: Pack 50 ml of TOYOPEARL SuperQ-650M resin in a chromatography column (diameter: 2.5 cm, length: 10 cm) (Tosoh Corporation, catalog number: 17227 ). - Acrylamide (Wako Pure Chemical Industries, Siyaku, catalog number: 016-00765 )
- N, N’-Methylenebisacrylamide (Nacalai Tesque, catalog number: 22402-02 )
- Ammonium persulfate (Wako Pure Chemical Industries, Siyaku, catalog number: 7727-54-0 )
- N, N, N’, N’-Tetramethylethylenediamine (Nacalai Tesque, catalog number: 33401-72 )
- Ammonium sulfate (Wako Pure Chemical Industries, Siyaku, catalog number: 019-03435 )
- Sodium chloride (Wako Pure Chemical Industries, Siyaku, catalog number: 198-01675 )
- Lactobacilli MRS Broth (MRS medium powder) (BD, catalog number: 288130 ) (see Recipes)
- 50 mM sodium phosphate buffer (pH 7.2) containing 1 mM EDTA and 1 mM dithiothreitol (see Recipes)
Note: Unless otherwise indicated, this buffer is used as the standard buffer throughout the purification procedures.- Sodium dihydrogenphosphate dihydrate (Wako Pure Chemical Industries,
Siyaku, catalog number: 192-02815 ) - Disodium hydrogenphosphate 12-water (Wako Pure Chemical Industries,
Siyaku, catalog number: 196-02835 ) - Ethylenediamine-N, N, N', N'-tetraacetic acid, disodium salt, dehydrate (EDTA)
(Dojindo Molecular Technologies, catalog number: N001 ) - Dithiothreitol (Nacalai Tesque, catalog number: 14128-04 )
- Sodium dihydrogenphosphate dihydrate (Wako Pure Chemical Industries,
- Red Sepharose CL-4B column (Ohshima and Sakuraba, 1986) (see Recipes)
Note: According to Recipes, prepare Red Sepharose CL-4B resin by attaching Reactive Red 120 (Sigma-Aldrich, catalog number: R0378-50G ) to Sepharose® CL-4B (Sigma-Aldrich, catalog number: CL4B200-100ML ). Pack 10 ml of Red Sepharose CL-4B resin in a chromatography column (diameter: 1.5 cm, length: 12 cm). - 1.5 M Tris-HCl buffer (pH 8.8) (see Recipes)
- 0.5 M Tris-HCl buffer (pH 6.8) (see Recipes)
- 2-Amino-2-hydroxymethyl-1, 3-propanediol (Tris base)
- Hydrochloric acid
- 2-Amino-2-hydroxymethyl-1, 3-propanediol (Tris base)
- Native-PAGE electrophoresis buffer (see Recipes)
- 2-Amino-2-hydroxymethyl-1, 3-propanediol (Tris base)
- Glycine (Wako Pure Chemical Industries, Siyaku, catalog number: 077-00735 )
- 2-Amino-2-hydroxymethyl-1, 3-propanediol (Tris base)
- 2x Native-PAGE gel loading buffer (see Recipes)
- L. otakiensis JCM 15040 obtained from Japan Collection of Microorganisms (JCM)
- Isoleucine 2-epimerase activity assay
- Pyridoxal-5’-phosphate (Nacalai Tesque, catalog number: 29606-74 )
- L-isoleucine (PEPTIDE INSTITUTE, catalog number: 2712 )
- Flavin adenine dinucleotide disodium salt (Nacalai Tesque, catalog number: 16010-06 )
- 4-Aminoantipyrine (Nacalai Tesque, catalog number: 01907-52 )
- Phenol (Wako Pure Chemical Industries, Siyaku, catalog number: 168-12721 )
- D-Amino acid oxidase from porcine kidney (Sigma-Aldrich, catalog number: A5222-200UN )
- Peroxidase (TOYOBO BIO CHEMICAL DEPT, catalog number: PE0-301 )
- 0.5 M sodium phosphate buffer (pH 8.0) (see Recipes)
- Sodium dihydrogenphosphate dihydrate
- Disodium hydrogenphosphate 12-water
- Sodium dihydrogenphosphate dihydrate
- Pyridoxal-5’-phosphate (Nacalai Tesque, catalog number: 29606-74 )
Equipment
- Purification of the isoleucine 2-epimerase
- Incubator (TITEC CORPORATION, model: BR-43FM )
- Centrifuge (Hitachi Koki Co., model number: CR 21F and TOMY SEIKO CO., model number: MX-300 )
- Multi-Beads shocker (Yasui Kikai Corporation)
- Electrophoresis apparatus
- Column chromatography equipment (see Representative data)
- Magnetic stirrer (ASONE Corporation, model: HS-50E )
- Two-way stopcock (Bio-Rad Laboratories, catalog number: 7328102 )
- Three-way stopcock (TERUMO CORPORATION, catalog number: TS-TR1K )
- Chromatography column (diameter: 2.5 cm, length: 10 cm) (Bio-Rad Laboratories, catalog number: 7372512 )
- Chromatography column (diameter: 1.5 cm, length: 12 cm) (Bio-Rad Laboratories, catalog number: 7321010 )
- Fraction tube (15 ml scale) (ASONE Corporation, catalog number: 2-8007-02 )
- Incubator (TITEC CORPORATION, model: BR-43FM )
- Isoleucine 2-epimerase activity assay
- Spectrophotometer (Shimadzu Scientific Instruments, model number: UVmini-1240 )
- Water bath (TOKYO RIKAKIKAI CO., model number: NTT-20S )
- Vortex (TAITEC CORPORATION, catalog number: 0061271-000 )
- Spectrophotometer (Shimadzu Scientific Instruments, model number: UVmini-1240 )
Procedure
文章信息
版权信息
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mutaguchi, Y. and Ohshima, T. (2015). Purification of a Protein Exhibiting Isoleucine 2-epimerase Activity from Lactobacillus otakiensis JCM 15040. Bio-protocol 5(20): e1632. DOI: 10.21769/BioProtoc.1632.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link