A Protocol to Depict the Proteolytic Processes Using a Combination of Metal–Organic Materials (MOMs), Electron Paramagnetic Resonance (EPR), and Mass Spectrometry (MS)
组合使用金属有机材料 (MOM)、电子顺磁共振 (EPR) 和质谱 (MS) 来描述蛋白水解过程的方案
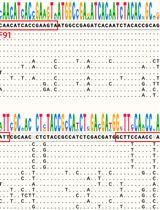
Proteolysis is a critical biochemical process yet a challenging field to study experimentally due to the self-degradation of a protease and the complex, dynamic degradation steps of a substrate. Mass spectrometry (MS) is the traditional way for proteolytic studies, yet it is challenging when time-resolved, step-by-step details of the degradation process are needed. We recently found a way to resolve the cleavage site, preference/selectivity of cleavage regions, and proteolytic kinetics by combining site-directed spin labeling (SDSL) of protein substrate, time-resolved two-dimensional (2D) electron paramagnetic resonance (EPR) spectroscopy, protease immobilization via metal–organic materials (MOMs), and MS. The method has been demonstrated on a model substrate and protease, yet there is a lack of details on the practical operations to carry out our strategy. Thus, this protocol summarizes the key steps and considerations when carrying out the EPR/MS study on proteolytic processes, which can be generalized to study other protein/polypeptide substrates in proteolysis. Details for the experimental operation and cautions of each step are reported with figures illustrating the concepts. This protocol provides an effective approach to understanding the proteolytic process with the advantages of offering time-resolved, residue-level resolution of structural basis underlying the process. Such information is important for revealing the cleavage site and proteolytic mechanisms of unknown proteases. The advantage of EPR, probing the target substrate regardless of the complexities caused by the proteases and their self-degradation, offers a practically effective, rapid, and easy-to-operate approach to studying proteolysis.Key features• Combining protease immobilization, EPR, spin labeling, and MS experimental methods allows for the analysis of proteolysis process in real time.• Reveals cleavage site, kinetics of product generation, and preference of cleavage regions via time-resolved SDSL-EPR.• MS confirms EPR findings and helps depict the sequences and populations of the cleaved segments in real time.• The demonstrated method can be generalized to other proteins or polypeptide substrates upon proteolysis by other proteases.Graphical overview