往期刊物2022
卷册: 12, 期号: 23
生物化学
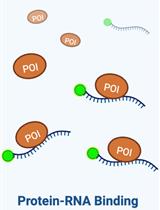
Assessing the in vitro Binding Affinity of Protein–RNA Interactions Using an RNA Pull-down Technique
利用 RNA Pull-down 技术评价蛋白质-RNA 相互作用的体外结合亲和力
癌症生物学
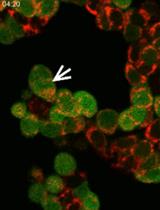
Fluorescence Time-lapse Imaging of Entosis Using Tetramethylrhodamine Methyl Ester Staining
使用四甲基罗丹明甲酯染色对内吞死亡进行荧光延时成像
免疫学
Improved Macrophage Enrichment from Mouse Skeletal Muscle
小鼠骨骼肌巨噬细胞富集的改善
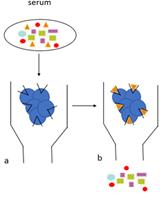
Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification
用蛋白G亲和纯化法从大量血清样品中纯化人类自身 IgG
医学
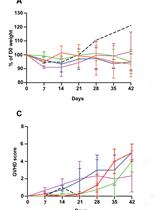
Modelling Graft-Versus-Host Disease in Mice Using Human Peripheral Blood Mononuclear Cells
使用人外周血单核细胞模拟小鼠移植物对抗宿主疾病
分子生物学
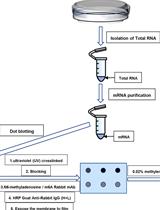
Analysis of N6-methyladenosine RNA Modification Levels by Dot Blotting
通过斑点印迹法分析 N6-甲基腺苷 RNA 修饰水平
神经科学
Infection of the Developing Central Nervous System of Drosophila by Mammalian Eukaryotic and Prokaryotic Pathogens
哺乳动物真核和原核病原体对果蝇发育中枢神经系统的感染
Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
条件性舔抑制:评估独立于小鼠运动活动的情境、线索和情境线索复合恐惧反应
植物科学
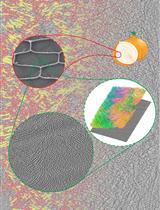
Focused Ion Beam Milling and Cryo-electron Tomography Methods to Study the Structure of the Primary Cell Wall in Allium cepa
用聚焦离子束铣削和低温电子断层扫描术方法研究洋葱原代细胞壁的结构
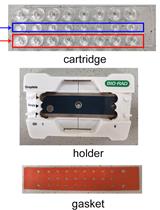
Measurement of Transgenes Copy Number in Wheat Plants Using Droplet Digital PCR
利用微滴数字 PCR 测量小麦植株中的转基因拷贝数