Advanced Search
A Simplified Hydroponic Culture of Arabidopsis
Published: Dec 20, 2018 DOI: 10.21769/BioProtoc.3121 Views: 11872
Edited by: Renate Weizbauer Reviewed by: Lusheng FanVikas Srivastava
Abstract
Hydroponic culture systems are widely used in research due to their intrinsic properties, such as easily altering nutrient composition, applying chemical treatments like metal, salt and hormones in the growth media, and collecting the root sample. Here, we describe a relatively easy and economic hydroponic culture system of the model plant Arabidopsis thaliana. It is worthy to note that this simple system can be adjusted and is suitable for other plant organisms.
Keywords: Hydroponic cultureBackground
Arabidopsis has been adopted as a model organism for a long time due to the significant features including its short life cycle, its well-annotated genome, easy transformation, and the availability of different types of mutants. Hydroponic culture has been considered as a very useful system in studying plant responses to nutrient or hormone changes since manipulation of the concentration or composition of mineral nutrients is feasible and phenotype observation of the morphology and architecture or sample collection are also convenient for diverse analyses (Hoagland and Arnon, 1941; Tocquin et al., 2003; Conn et al., 2013). However, the small size, the rosette growth habit, and the sensitivity of shoot apical meristem to flooding make the hydroponic growth of Arabidopsis difficult. Several hydroponic culture systems have been developed for the growth of Arabidopsis (Gibeaut et al., 1997; Arteca and Arteca, 2000; Schlesier et al., 2003; Tocquin et al., 2003; Norén et al., 2004; Smeets et al., 2008; Conn et al., 2013). Some of the systems have often been designed for a specific purpose and could not be suitable for various experimental purposes (Arteca and Arteca, 2000; Schlesier et al., 2003; Norén et al., 2004); some of the systems have to use rockwool or sponge, which could prevent the collection of root tissues near the root-shoot junction and risk the shoot apical meristem in a flooding stress (Gibeaut et al., 1997; Smeets et al., 2008); some of the systems require specialized materials like seed holder, raft float and container (Arteca and Arteca, 2000; Tocquin et al., 2003; Conn et al., 2013). In addition, algae growing is a common problem when rockwool, sponge or agar-based plugs are used.
In 2013, Conn et al. developed an Arabidopsis hydroponic system by germinating seeds on the pierced cap of 1.5 ml microcentrifuge tube in the germination solution, and then transferring the seedlings to the modified 50 ml Falcon tubes which are placed in an aerated large container filled with the basal growth solution. This system has some advantages of preventing algae growth and ensuring the harvest of the whole root system. However, it is relatively complex to prepare some materials, such as the pierced lids of 50 ml Falcon tubes and two different nutrient solutions (the germination solution and the basal growth solution). Here, we simplified Conn’s method by using the materials and equipment that can be easily found in the laboratory or cheaply bought online, like the 0.5 ml microcentrifuge tube, recycled 1 ml pipette tip box, plastic bucket, and the air pump. Particularly, we only use ½ Hoagland nutrient solution during the whole life cycle of the plant to simplify the making of nutrient solutions. The construction of the hydroponic culture system we described here should be much simpler and cheaper compared with the previous systems (Tocquin et al., 2003; Conn et al., 2013). Our system also has the advantages of preventing algae growth, ensuring the harvest of the whole root system and maintaining high uniformity of the plants. In our hydroponic system, the plant can healthily complete the whole life cycle and can be treated and sampled at different growth stages according to research goals. The root system can be harvested totally without any damage. The hydroponic system can be used for various treatments like nutrient deficiencies, hormonal and chemical treatments, for shoot and root growth observation and measurement, and for shoot and root sample collection that can be used for various analyses at physiological, biochemical, metabolic, cellular and molecular levels. The size of a container used for plant growth can be adjusted based on the experimental goal and budget. For example, big bucket can be switched to 1 ml pipette tip box in the hydroponic culture system to save the cost of nutrient solution and chemical treatments if a large amount of plant materials is not necessarily required.
Materials and Reagents
- 0.5 ml flat-top microcentrifuge tube
- Single edge blade (Personna, 0.23 mm)
- 1 ml pipette tip boxes with racks and covers
- Scotch tape
- Air tubing (3/16" in diameter) (Elemental Solutions, catalog number: ECAT2010) (Figure 1B)
- Arabidopsis thaliana seeds
- 70% ethanol
- Deionized water
- Sterilized dH2O
- Agar (Sigma-Aldrich, catalog number: A1296)
- Potassium nitrate (KNO3) (Fisher Bioreagents, catalog number: BP368-500)
- Calcium nitrate tetrahydrate (Ca(NO3)2•4H2O) (Fisher Chemical, catalog number: C109-500)
- Ammonium nitrate (NH4NO3) (Fisher Chemical, catalog number: A676-500)
- Magnesium sulfate heptahydrate (MgSO4•7H2O) (Fisher Chemical, catalog number: M80-500)
- Potassium dihydrogen phosphate (KH2PO4) (Alfa Aesar, catalog number: AAA1214236)
- NaFe(III) EDTA [conjugated using FeSO4•7H2O (Honeywell, catalog number: F7002-500G) and EDTA Na2•2H2O (Fisher chemical, catalog number: O2793-500)]
- Boric acid (H3BO3) (Fisher Chemical, catalog number: A73500)
- Manganese(II) chloride tetrahydrate (MnCl2•4H2O) (Sigma, catalog number: M3634-500G)
- Zinc sulfate heptahydrate (ZnSO4•7H2O) (Awresco, catalog number: 97061-324)
- Copper(II) sulfate pentahydrate (CuSO4•5H2O) (VWR International, catalog number: BDH9312-500G)
- Sodium molybdate dihydrate (Na2MoO4•2H2O) (Alfa Aesar, catalog number: A19222)
- ½ Hoagland's nutrient solution (pH 5.6) (see Recipes)
Equipment
- Scissors
- Single-channel pipettes (200 µl, 1 ml)
- Plastic buckets (one gallon, black) and bucket lids with seven holes drilled (six holes for holding plants, one hole for air tubing)
Each hole is about 7.5 mm in diameter, and can be drilled using a driller with a bit of 0.25" (https://www.amazon.com/Gallon-Black-Plastic-Pail-Bottom/dp/B06XPQ61X3) (Figure 1A) - Air pumps (Elemental, 380 gph) (Elemental Solutions, catalog number: EOP246) (Figure 1B)
- Air stone (2" in diameter and compatible with 3/16" air tubing) (Elemental Solutions, catalog number: EOCS202) (Figure 1B)
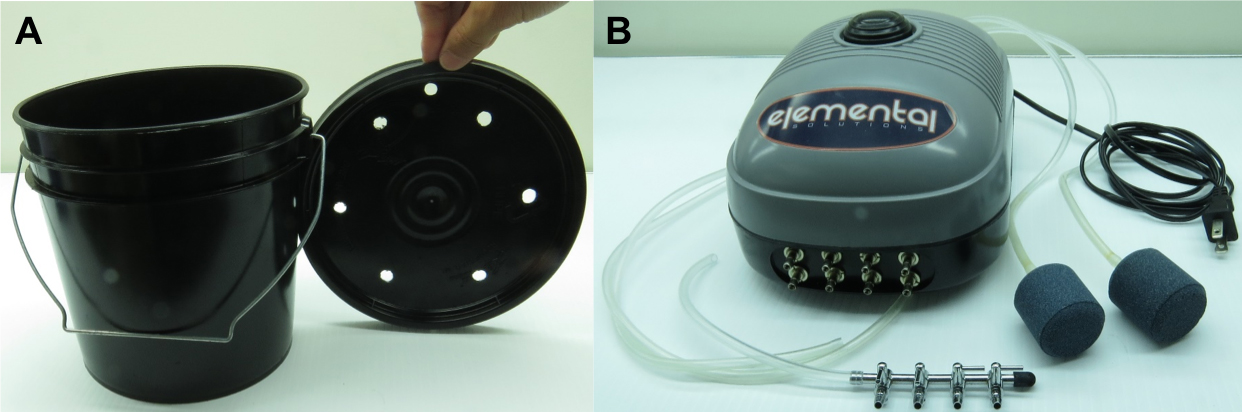
Figure 1. Parts of the equipment used for hydroponic culture of Arabidopsis. A. Black bucket (one gallon) and lid with seven holes drilled. B. Air pump, air tubing, air stone, and four-way outlet valve. - Four-way outlet valve (https://www.amazon.com/dp/B01N2K46XR/ref=sspa_dk_detail_1?psc=1&pd_rd_i=B01N2K46XR&pd_rd_wg=Lf7RJ&pd_rd_r=QDPMR7T6RBNYDGKFAB0G&pd_rd_w=roaMV) (Figure 1B)
- 24 h plug-in mechanical timer (https://www.amazon.com/Century-Plug-Mechanical-Timer-Grounded/dp/B00MVFF59S/ref=sr_1_9?ie=UTF8&qid=1541631923&sr=8-9&keywords=24-hour+timer+heavy+duty)
- Polyethylene tank (25 gallons, 19" Diameter x 26" High) (United States Plastic, Tamco® Industries, catalog number: 3491)
- Polyethylene tank cover (25 gallons) (United States Plastic, Tamco® Industries, catalog number: 3021)
Procedure
Category
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
