Advanced Search
Dissection and Whole Mount Staining of Retina from Neonatal Mice
Published: Oct 5, 2018 DOI: 10.21769/BioProtoc.3034 Views: 7610
Abstract
Here we provide a detailed protocol for whole mount staining of mouse retina. This protocol was used to analyze retinal angiogenesis in newborn mice (Sawaguchi et al., 2017) by modifying the original protocols (Powner et al., 2012; Tual-Chalot et al., 2013). This protocol can also be used for whole mount staining of adult retina.
Keywords: RetinaMaterials and Reagents
- 1 ml Pipette tips (Thermo Fisher Scientific, QSP, catalog number: 111-N-Q )
- 100 μl Pipette tips (Thermo Fisher Scientific, QSP, catalog number: TTW110RLNS-Q )
- Microtube (INA•OPTIKA, BIO-BIK, catalog number: ST-0150F )
- Postnatal day 5 (P5) or P15 mouse
- 4% paraformaldehyde (PFA) (Wako Pure Chemical Industries, catalog number: 163-20145 )
- Methanol (Wako Pure Chemical Industries, catalog number: 137-01823 )
- Donkey (ImmunoBio Science, catalog number: IHR-8135 ) or goat serum (Wako Pure Chemical Industries, catalog number: 143-06561)
- Cy3- or FITC-conjugated anti-αSMA (clone 1A4) (Sigma-Aldrich, catalog number: F3777 )
- CF®488A-conjugated Streptavidin (Biotium, catalog number: 29034 ) or Dylight 649-conjugated streptavidin (Vector Laboratories, catalog number: SA-5649 )
- Dylight 594-conjugated IB4 (Vector Laboratories, catalog number: DL-1178 )
- Vectashield® antifade mounting medium (Vector Laboratories, catalog number: H-1000 )
- Griffonia Simplicifolia IB4, Biotinylated (Vector Laboratories, catalog number: B-1105 )
- Na2HPO4 (Wako Pure Chemical Industries, catalog number: 196-02835 )
- KH2PO4 (Sigma-Aldrich, catalog number: 24-5260 )
- NaCl (Wako Pure Chemical Industries, catalog number: 191-01665 )
- KCl (Wako Pure Chemical Industries, catalog number: 163-03545 )
- Triton X-100 (Sigma-Aldrich, catalog number: T9284 )
- Bovine serum albumin (BSA) (Equitec-Bio, catalog number: BAC62 )
- Phosphate buffered saline (PBS), pH 7.4 (see Recipes)
- PBSTX (see Recipes)
- Perm/Block solution (see Recipes)
Equipment
- Pipettes (various sizes) (Gilson)
- Tweezers (Fine Science Tools, model: Dumont #5 )
- Dissecting scissors (Fine Science Tools, catalog number: 15003-08 )
- Dissecting microscope (Olympus, model: SZX7 )
- Tube rotator (TAIYO ELECTRIC, model: RT-50 )
- Fluorescence microscope (Nikon Instruments, model: TiE-A1R-KT5 )
Procedure
Note: All experimental procedures were conducted in accordance with the Guidelines for Animal Experimentation in Nagoya University Graduate School of Medicine and Japanese Government Animal Protection and Management Law.
- Fix eyes from the postnatal day 5 (P5) or P15 mouse in 4% paraformaldehyde (PFA) at room temperature (RT) for 15 min.
Note: For detecting filopodia at the vascular front, eyes are fixed for 2 h on ice. - Dissect retinas in PBS using tweezers and dissecting scissors under a microscope (Figure 1).
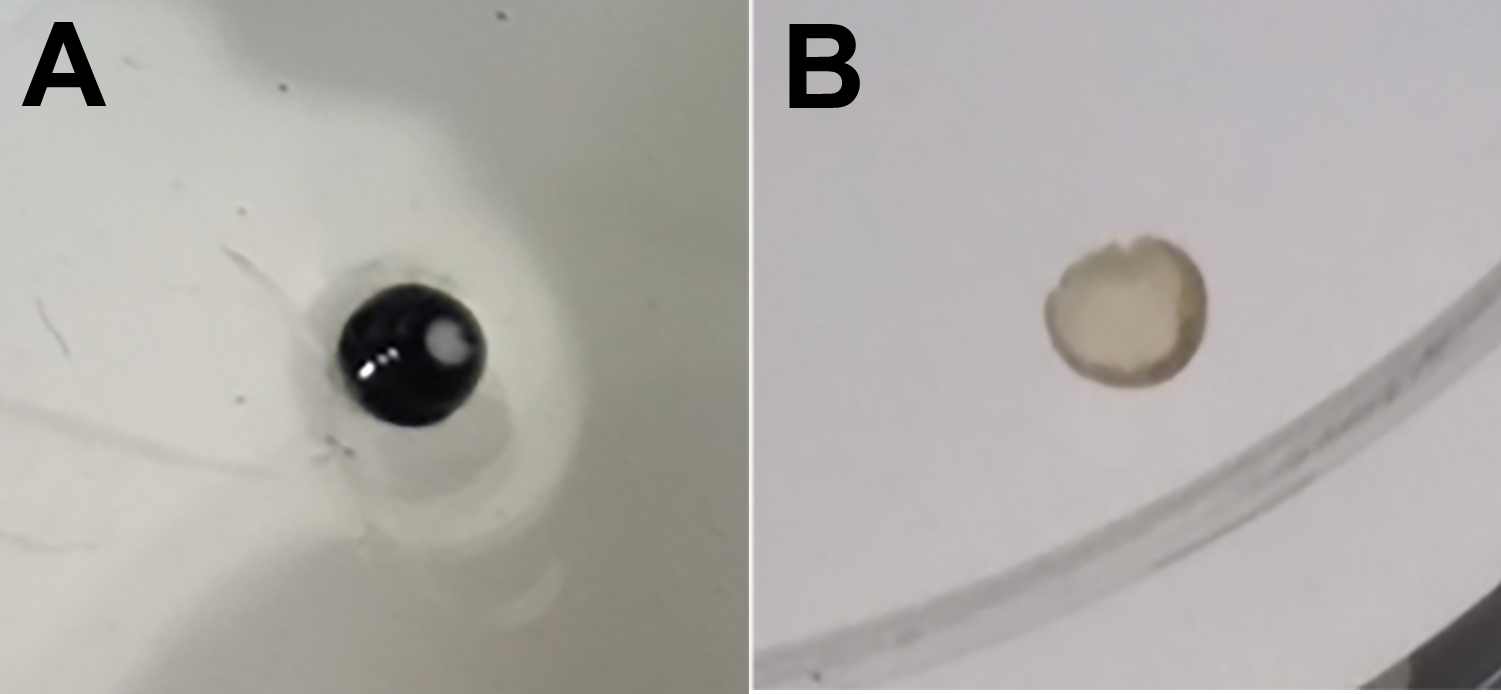
Figure 1. Dissection of retinas in PBS using tweezers - Prepare flat retinas by dropping cold methanol onto dissected retinas (Figure 2).
Note: Retina becomes flat by fixing with cold methanol.
Figure 2. Preparation of flat retina by dropping cold methanol - Incubate retinas in Perm/Block solution supplemented with 5% donkey or goat serum for 1 h at RT using a tube rotator.
- Incubate retinas with Cy3- or FITC-conjugated anti-αSMA and biotin-IB4 in Perm/Block solution overnight at 4 °C using a tube rotator.
- Wash retinas 4 times with PBSTX each for 10 min at RT.
- Incubate retinas with Dylight 649-conjugated streptavidin or CF®488A-conjugated streptavidin in Perm/Block solution for 2 h at 4 °C.
Note: Retinas can be directly labeled with Dylight 594-conjugated IB4. - Wash retinas for 4 times with PBSTX each for 10 min at RT and rinse with PBS.
- Mount retinas using Vectashield® antifade mounting medium and observe under a TiE-A1R-KT5 microscope (Figure 3).
Note: A coronal view cannot be acquired as it is whole mount staining of retina.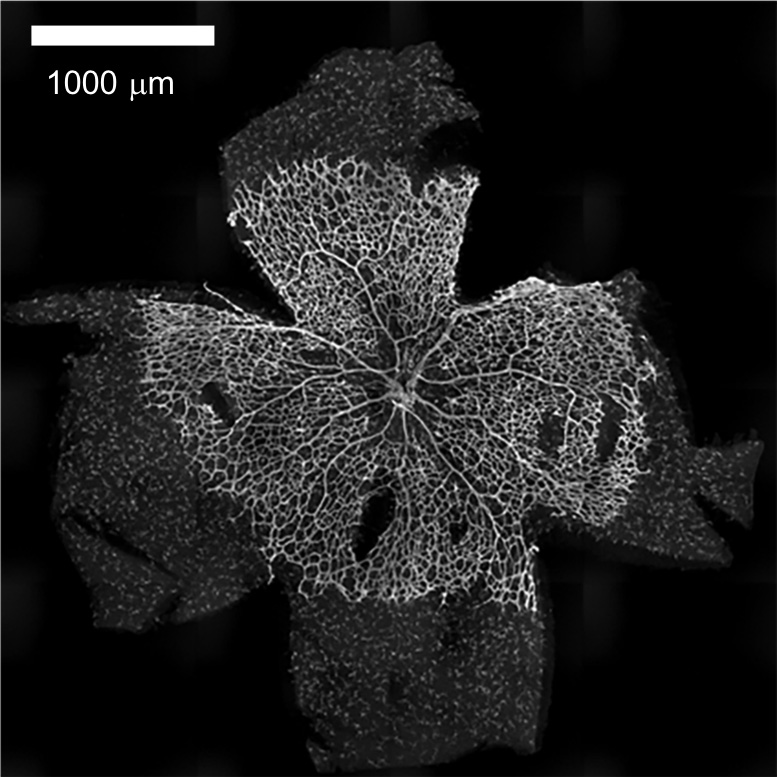
Figure 3. Whole staining of P5 retina using IB4 lectin
Recipes
- Phosphate buffered saline (PBS), pH 7.4
10 mM Na2HPO4
1.8 mM KH2PO4
137 mM NaCl
2.7 mM KCl - PBSTX
0.3% Triton X-100
PBS, pH 7.4 - Perm/Block solution
PBS, pH 7.4
0.3% Triton X-100
0.2% bovine serum albumin (BSA)
Acknowledgments
We thank N. Toida (Nagoya Univ) for technical support. This protocol is modified from the previously published article (Sawaguchi et al., 2017). This work was supported by Japan Society for the Promotion of Science grants # JP15K15064 to TO and MO, #JP26110709 to TO, #JP26291020 to TO, #JP15K18502 to MO, #JP16J00004 to MO; Takeda Science Foundation to TO; Japan Foundation for Applied Enzymology to TO; YOKOYAMA Foundation for Clinical Pharmacology #YRY-1612 to MO. The authors declare no conflict of interest.
References
- Powner, M. B., Vevis, K., McKenzie, J. A., Gandhi, P., Jadeja, S. and Fruttiger, M. (2012). Visualization of gene expression in whole mouse retina by in situ hybridization. Nat Protoc 7(6): 1086-1096.
- Sawaguchi, S., Varshney, S., Ogawa, M., Sakaidani, Y., Yagi, H., Takeshita, K., Murohara, T., Kato, K., Sundaram, S., Stanley, P. and Okajima, T. (2017). O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. Elife 6: e24419.
- Tual-Chalot, S., Allinson, K. R., Fruttiger, M. and Arthur, H. M. (2013). Whole mount immunofluorescent staining of the neonatal mouse retina to investigate angiogenesis in vivo. J Vis Exp (77): e50546.
Ogawa and Okajima. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Category
Developmental Biology > Morphogenesis > Cell structure
Cell Biology > Cell imaging > Fixed-tissue imaging
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

