Advanced Search
Screening and Genetic Analysis of Ethylene-Response Mutants in Etiolated Rice Seedlings
*Contributed equally to this work Published: Sep 5, 2018 DOI: 10.21769/BioProtoc.3001 Views: 4718
Abstract
Ethylene, the simplest gaseous plant phytohormone, is involved in the control of rice growth and development processes, but the mechanism of ethylene regulating these pathways remains unclear in rice. Recent studies have found that ethylene-signaling pathway is conserved but different between rice and Arabidopsis. The forward genetic analysis is an essential and efficient method to reveal fully the mechanism of ethylene signaling in rice plants. Here we provide a protocol of genetic analysis of rice ethylene-response mutant, including screening ethylene-response mutants, treatment with ethylene and a chemical reagent, and ethylene-responsiveness gene expression analysis.
Keywords: RiceBackground
The key molecular elements of ethylene-signaling pathway have been identified by molecular genetics and genomic approaches in Arabidopsis (Guo and Ecker, 2004). Ethylene plays important roles in rice growth, development, and environmental adaptation, including coleoptile and shoot elongation, aerenchyma development, and submergence response (Xu et al., 2006; Fukao and Bailey-Serres, 2008; Ma et al., 2010). Rice may have a distinct mechanism of ethylene-signaling pathway due to the different ethylene-regulated biological processes in rice and Arabidopsis (Yang et al., 2015a). The forward genetic analysis is essential to reveal fully the mechanism of ethylene signaling pathway in rice. So, it is prerequisite to develop an efficient method to screen ethylene-response mutants in rice. Genetic screens that are based on the triple-response have been extensively conducted on Arabidopsis (Johnson and Ecker, 1998; Stepanova and Ecker, 2000). However, the genetic screens in rice have been hamper owing to the lack of ethylene-response phenotypes.
Ethylene promotes rice coleoptile elongation of dark-grown seedlings (Ku et al., 1970). In our experimental conditions, root elongation can be inhibited by ethylene in etiolated rice seedlings. Over the past decade, based on the double-response of etiolated rice seedling, including ethylene-induced coleoptile growth promotion and root growth inhibition, several unique rice mutants have been identified. We named these ethylene-response mutants maohuzi (mhz) (Ma et al., 2013). Loss-of-function of MHZ7/OsEIN2 rice plants display insensitive to ethylene, including ethylene-insensitive coleoptile elongation and root growth. Conversely, MHZ7/OsEIN2-overexpression transgenic lines display ethylene hypersensitivity (Ma et al., 2013). Characterization of MHZ6/OsEIL1 revealed that MHZ6/OsEIL1 and its homolog gene OsEIL2 have functional diversification in rice ethylene response (Yang et al., 2015b). Identifications of MHZ4/ABA4 (Ma et al., 2014) and MHZ5/CRTISO (Yin et al., 2015) revealed that ethylene inhibits root growth requiring ABA pathway in rice, which is different with that in Arabidopsis. The study of MHZ2/SOR1 provides a candidate mechanism that auxin acts downstream to modulate ethylene inhibition of root growth in etiolated rice seedlings (Chen et al., 2018). The research of MHZ3 found that ethylene-induced MHZ3 stabilizes MHZ7/OsEIN2 and impeding protein ubiquitination to facilitate ethylene signaling pathway (Ma et al., 2018). Here, we describe the method in detail for genetic analysis of ethylene response mutants in etiolated rice seedlings. This protocol can also be used in other monocotyledonous plants to detect ethylene response (Yang et al., 2015a). Besides, the longer mesocotyl and coleoptile mutant gaoyao1(gy1) was also discovered by this method (Xiong et al., 2017).
Materials and Reagents
- Syringes with needle: 2-, 5-, and 60-ml capacity
- Petri dish, 60 mm (Corning, PYREX®, catalog number: 3160-60 )
- Airtight plastic and cups:
- 5.5 L box (Lock & Lock, catalog number: HPL 836 ) matched with the 12-lattice sieve
- 520 ml (Lock & Lock, catalog number: HPL 931N ) cup matched with the wire mash
Note: The capacity of the cup is 520 ml when covered with its lid. - 20 L box (FUDOGIKEN, catalog number: 112025 ) matched with the 100-lattice sieve
Note: There is a hole drilled into to the side of each box and fitted with a silicon rubber stopper; Cups with lid and silicon rubber stoppers (Figure 1).
- Plant seeds: Wild-type, mutants, varieties or transgenic lines
- Ethylene gas
- 1-MCP (1-Methylcyclopropene) (LuNuo Bio-Technology, Maxfresh)
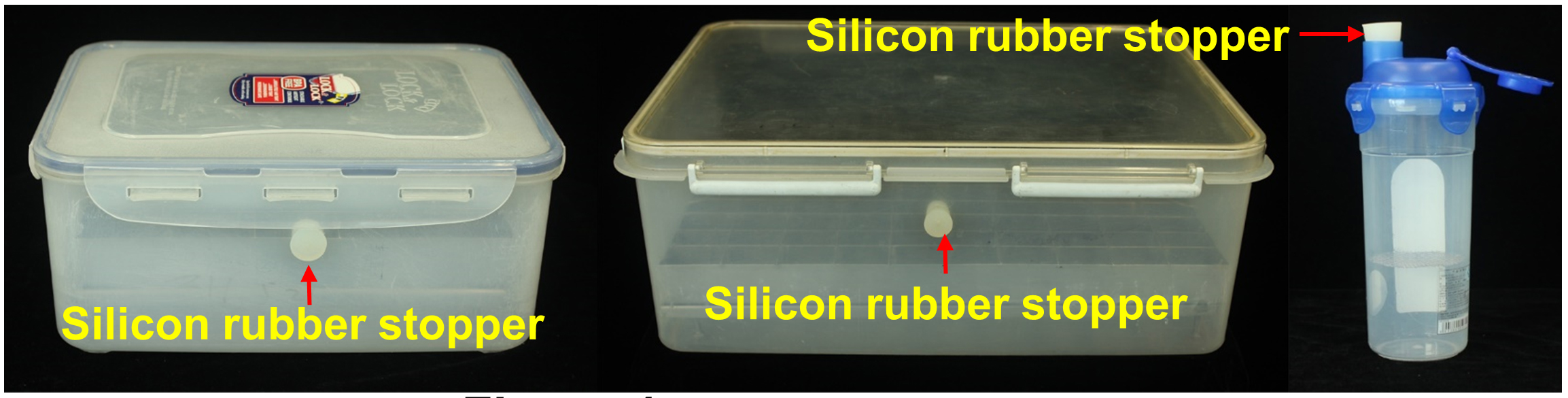
Figure 1. Airtight plastic boxes and cups. Each of the containers has a fitted silicon rubber stopper in order to inject ethylene gas.
Equipment
- Beaker (BOMEX, 250 ml or 500 ml, depends on the number of seeds)
- Dim green light source (ordinary commercial green light is OK)
- Silicon rubber stopper (Laboran, catalog numbers: 9-860-03 , 9-860-06 , 9-860-09 , 9-860-11 and so on)
- Stainless sieves (custom made) and wire mesh:
- 12-lattice sieve (240 mm length x 180 mm width x 33 mm height) for phenotype analysis
- 62 mm diameter wire mesh, and 100-lattice sieve (400 mm length x 300 mm width x 33 mm height) for mutant screening, positive seedlings selection and genetic mapping
- Graduated cylinders: 1,000-, 2,000-, and 4,000-ml capacity
- Incubators for culturing rice seedlings set at 28 °C and 37 °C
- Drying Oven
- Watering can
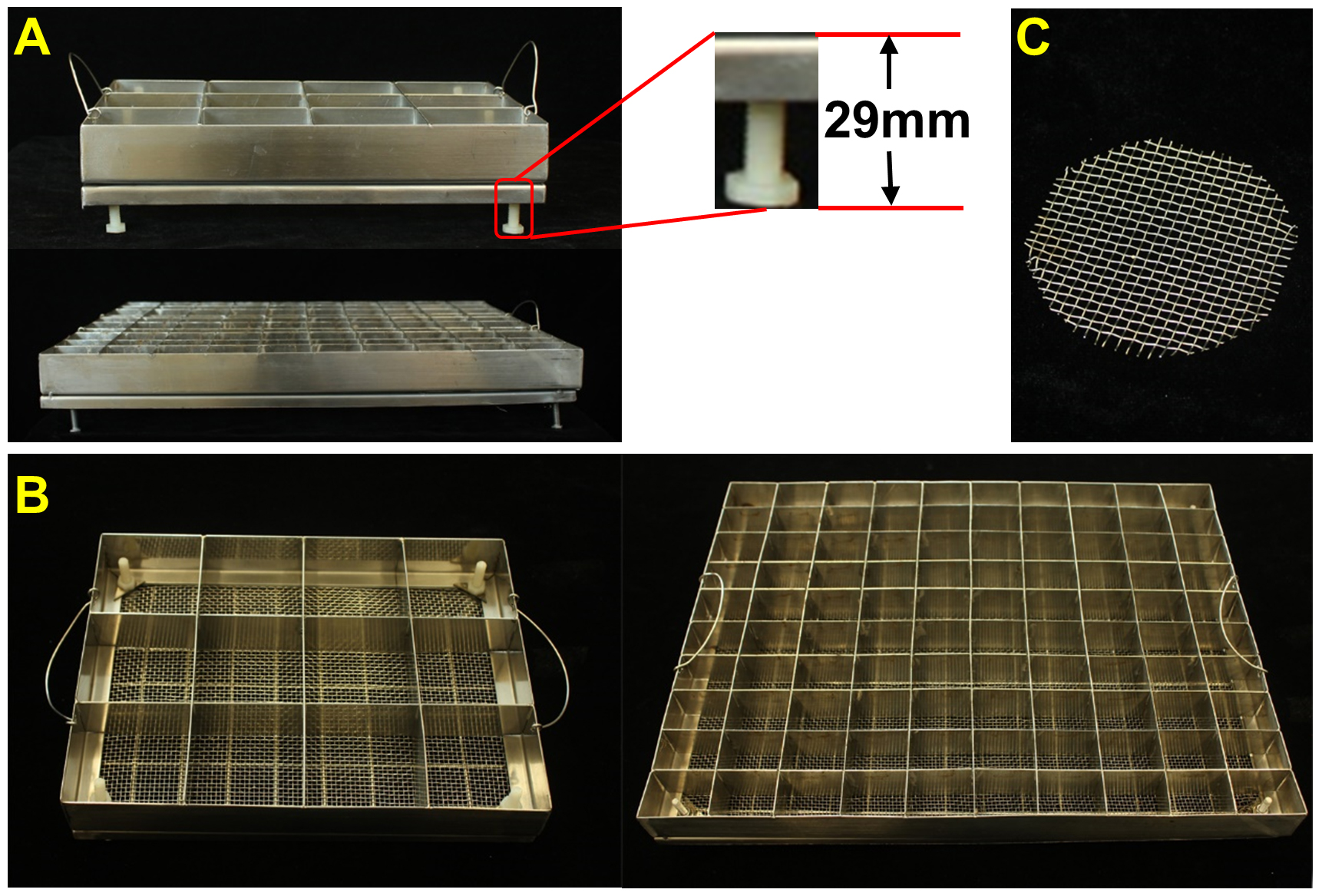
Figure 2. 12-lattice sieve, 100-lattice sieve, and wire mesh. A. Side view of the sieves. B. Top view of the sieves. C. 62 mm diameter wire mesh.
Procedure
Category
Plant Science > Plant physiology > Phenotyping
Plant Science > Plant molecular biology > Genetic analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

