Abstract
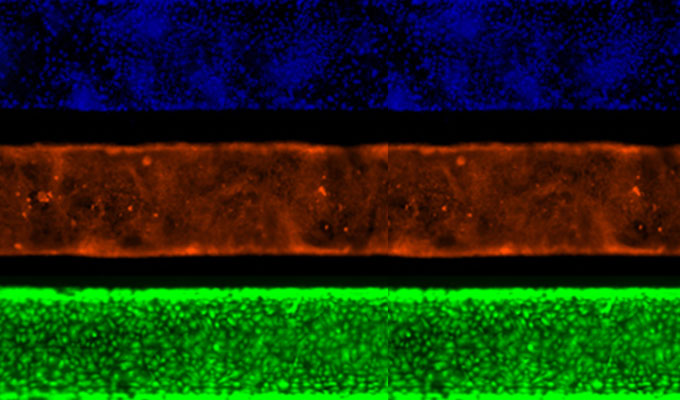
Live-cell fluorescence microscopy provides powerful insights into dynamic cellular processes but poses the challenge of maintaining cell viability during extended imaging sessions. This webinar presents an optimized protocol that carefully balances spatial resolution, temporal depth, and cell preservation using red and near-infrared viable probes combined with advanced imaging strategies to minimize phototoxicity.
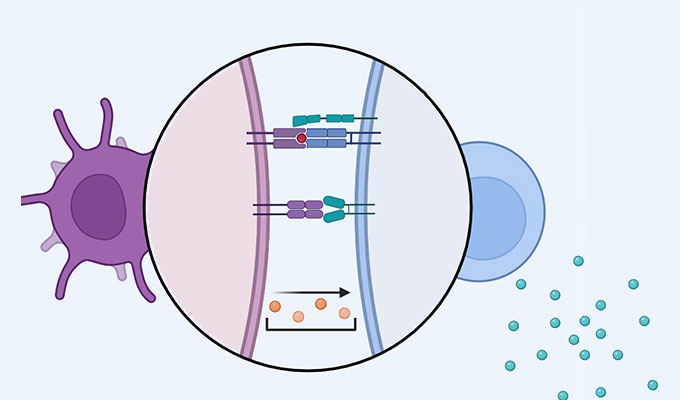
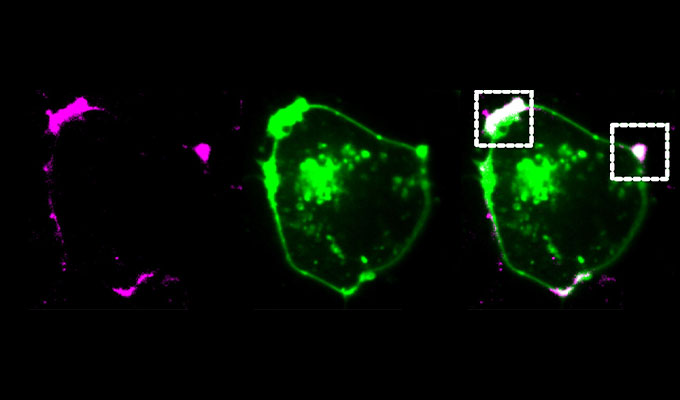
The session will guide viewers through the full workflow, including probe selection, optimized concentrations, and incubation conditions for effective multi-labeling. The protocol employs advanced confocal laser scanning microscopy with hybrid detectors and a tunable white laser, integrated with fast fluorescence lifetime imaging (FLIM) and stimulated-emission-depletion (STED) nanoscopy to enable long-term imaging with high temporal and spatial resolution.
Additionally, lifetime-based approaches such as dye unmixing, environmental sensitivity analysis, and image denoising are utilized to enhance signal clarity and support flexible multiplexing. These combined techniques allow researchers to capture high-quality, long-duration live-cell and subcellular dynamics with minimal light-induced stress.
This webinar will walk participants through each critical step—from labeling to image acquisition and processing—demonstrating how FLIM and STED can be effectively applied for reproducible and minimally invasive live-cell imaging.
Highlights
- Strategies to minimize phototoxicity using red and near-infrared viable dyes.
- Optimized probe concentration and incubation for multi-labeling.
- Long-duration time-lapse imaging using advanced confocal microscopy with white laser and hybrid detectors.
- Integration of fast FLIM for lifetime-based multiplexing and dye unmixing.
- Combining STED with FLIM to improve signal-to-noise ratio and spatial resolution.
Speaker

Magalie Bénard, Ph.D.
Research Engineer, INSERM
Dr. Magalie Bénard is a senior research engineer at INSERM and co-director of the PRIMACEN (Plate-forme de Recherche en Imagerie Cellulaire de Norm...
View more
Moderator

Christophe Chamot
Research Engineer, HeRacLeS Research Infrastructure, INSERM
With over 25 years of experience in academia, industry, and scientific platforms, Christophe Chamot specialises in advanced imaging techniques, pri...
View more
Keywords
Live-cell imaging, Fluorescence lifetime, Phototoxicity reduction, Confocal microscopy, Fast FLIM, STED
References
Bénard, M., Chamot, C., Schapman, D., Lebon, A. and Galas, L. (2025). Combined FLIM, Confocal Microscopy, and STED Nanoscopy for Live-Cell Imaging. Bio-protocol 15(4): e5202. DOI: 10.21769/BioProtoc.5202.
Bénard, M., Chamot, C., Schapman, D., Debonne, A., Lebon, A., Dubois, F., Levallet, G., Komuro, H. and Galas, L. (2024). Combining sophisticated fast FLIM, confocal microscopy, and STED nanoscopy for live-cell imaging of tunneling nanotubes. Life Sci Alliance. 7(7): e202302398. https://doi.org/10.26508/lsa.202302398
Galas, L., Gallavardin, T., Bénard, M., Lehner, A., Schapman, D., Lebon, A., Komuro, H., Lerouge, P., Leleu, S., Franck, X., et al. (2018). “Probe, Sample, and Instrument (PSI)”: The Hat-Trick for Fluorescence Live Cell Imaging. Chemosensors. 6(3): 40. https://doi.org/10.3390/chemosensors6030040
Bénard, M., Schapman, D., Chamot, C., Dubois, F., Levallet, G., Komuro, H. and Galas, L. (2021). Optimization of Advanced Live-Cell Imaging through Red/Near-Infrared Dye Labeling and Fluorescence Lifetime-Based Strategies. Int J Mol Sci. 22(20): 11092. https://doi.org/10.3390/ijms222011092
Do you have a question about this webinar?
Post your question, and we'll invite the webinar speaker to respond. You're welcome to join the discussion by answering or commenting on questions ( Note: Not all questions, especially those not directly relevant to the webinar topic, may be answered by the speaker. ).
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question.
19 Q&A