- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Airway Responsiveness in the Anesthetized Mouse
Published: Vol 3, Iss 7, Apr 5, 2013 DOI: 10.21769/BioProtoc.645 Views: 12914

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2553 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2593 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 4027 Views
Abstract
Airway hyperresponsiveness to methacholine is an important characteristic of asthma. Many devices can be used to measure airway responsiveness in the mouse but it is well established that the invasiveness of a measurement technique is correlated with its precision and reproducibility. This protocol describes how to measure airway responses to aerosolized methacholine in anesthetized, tracheotomized, and mechanically ventilated mice using the forced oscillation technique with FlexiVent® (SCIREQ). This is a computer-controlled precision piston pump that can intersperse mechanical ventilation with volume and pressure controlled manoeuvres to obtain accurate, reproducible measurement of respiratory mechanics.
Keywords: Airway responsivenessMaterials and Reagents
- Sterile saline (0.9% NaCl) (B. Braun)
- Acetyl-β-methylcholine chloride, powder (Sigma-Aldrich, catalog number: A-2251 )
- Drierite (Sigma-Aldrich, catalog number: 238988 )
- Sodium Pentobarbital (5.47%) (CEVA)
- Xylazine (2%) (Rompun®, Bayer)
- Cotton wire n50 (DMC, catalog number: 334A/50 )
Equipment
- FlexiVent® system (SCIREQ Technologies) composed of the following modules: FV-M1 (1.8 ml), FV-PP-M1 , FV-XC , FV-EC , FV-BU and Aeroneb® Lab nebuliser system associated with Aeroneb® Pro (AG-AL-1000)
- "1 ml" sterile syringes (Terumo, catalog number: SS+01T1 )
- "25-gauge 0.5 mm" sterile needles (Terumo, catalog number: NN-2516R )
- 18-gauge metal needle (SCIREQ Technologies, catalog number: 73-0019 )
Procedure
Summary of the protocol to measure airway responsiveness to methacholine:
- Mice are weighed and anesthetised i.p. with xylasine and pentobarbital.
- Mice are tracheotomised and a 18-gauge metal needle is inserted into the trachea and connected to the FlexiVent® computer-controlled small animal ventilator.
- Baseline values are measured.
- Saline and then methacholine are aerosolized in the ventilator circuit.
- Airway measurements are performed with the FlexiVent® protocol.
- Mice are disconnected at the end to ensure that they breathe normally again without the aid of the ventilator.
- Anaesthesia
- Bring xylasine and pentobarbital solutions to room temperature (18-25 °C), and place methacholine and saline solutions on ice.
- Weigh the mouse.
- Hold the mouse in your hand by the dorsal skin so that its head is up and its rear legs are down. Maintain its tail with fingers.
- Use "1 ml" syringes and "25-gauge" needles to inject solution and administrate 6 ml/kg of the xylasine solution intraperitoneally (i.p.).
- Isolate the mouse in another cage. Wait for 10 min.
- Hold the mouse in your hand by the dorsal skin so that its head is up and its rear legs are down. Maintain its tail with fingers.
- Use another "1 ml" syringe and a "25-gauge" needle to inject 6 ml/kg of pentobarbital i.p.
- Replace the mouse in the cage. Wait for 10 min.
- Bring xylasine and pentobarbital solutions to room temperature (18-25 °C), and place methacholine and saline solutions on ice.
- Tracheotomy
- Check that the mouse is profoundly anesthetised.
- Dissect and expose the trachea, place a cotton wire under the trachea, insert the 18-gauge metal needle into the trachea and tie a knot around the trachea with the cotton wire in order to hold the needle in place.
- Connect the needle (i.e. connect the mouse) to the computer-controlled small animal ventilator (FlexiVent®).
- Follow the "Mouse_AN_v5.2.ext" standard protocol supplied with the FlexiVent®. This protocol consists of 12 cycles of 2 perturbations (snapshot and Prime-8) after 10 sec of nebulization, and allows to measure the resistance, elastance and compliance data.
- Check that the mouse is profoundly anesthetised.
- Measurement processes and methacholine nebulization
- Start ventilation when the mouse is connected.
- Start the protocol.
- Add saline in the nebuliser for the first nebulization step (10 sec) and proceed to the 12 perturbations cycles.
- Rinse the nebuliser 3 times with deionized water (eliminate solution with a syringe and replace it with water, repeat 3 times).
- Add methacholine in the nebuliser for the second nebulisation step (10 sec) and proceed to the 12 perturbations cycles.
- Stop the experiment and stop the ventilation.
- Disconnect immediately the 18-gauge metal needle from the apparatus. The mouse should breathe again normally, indicating it was alive during the measures. Then, euthanize the mouse. If the mouse is dead, data should be eliminated.
- Rinse the nebulizer 3 times with deionized water.
- Begin a new analysis session with the following mouse.
- Start ventilation when the mouse is connected.
- Data analysis
- Export data from the Scireq FlexiVent® V5.2 program to an Excel file
- For each saline or methacholine nebulization, the peak response is calculated as the mean of the three maximal values (in red on the figure here below), and used for calculation of airway resistance and compliance. Airway resistance is expressed as cm H2O.s.ml-1 and airway compliance as ml.cm H2O-1.
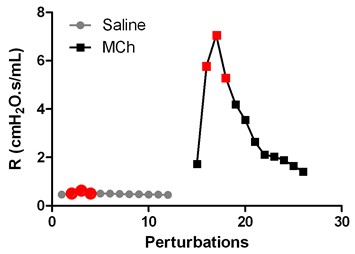
Figure 1.
- Export data from the Scireq FlexiVent® V5.2 program to an Excel file
Recipes
- Prepare a solution of methacholine containing 50 mg/ml methacholine (0.26 M) diluted in sterile saline. Methacholine must be prepared extemporaneously.
- Freshly prepare a solution of xylasine containing 0.25% xylasine diluted in sterile saline and a solution of pentobarbital containing 0.9% pentobarbital.
Acknowledgments
This protocol was adapted from a previously published paper: Reber et al. (2012). FD was supported by the “fond de dotation recherche en santé respiratoire”, call for tenders 2010.
References
- Reber, L. L., F. Daubeuf, M. Plantinga, L. De Cauwer, S. Gerlo, W. Waelput, S. Van Calenbergh, J. Tavernier, G. Haegeman, B. N. Lambrecht, N. Frossard and K. De Bosscher (2012). A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J Immunol 188(7): 3478-3487.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Daubeuf, F., Reber, L. and Frossard, N. (2013). Measurement of Airway Responsiveness in the Anesthetized Mouse. Bio-protocol 3(7): e645. DOI: 10.21769/BioProtoc.645.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









