- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy
Published: Vol 12, Iss 22, Nov 20, 2022 DOI: 10.21769/BioProtoc.4549 Views: 1929
Reviewed by: Kristin L. ShinglerWenn-Chyau LeeLucy XieAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sex-specific Separation of Plasmodium falciparum Gametocyte Populations
Melanie C. Ridgway [...] Alexander G. Maier
Jun 5, 2021 3889 Views

Plasmodium cynomolgi Berok Growth Inhibition Assay by Thiol-reactive Probe Based Flow Cytometric Measurement
Jessica Jie Ying Ong [...] Jin-Hee Han
Sep 5, 2021 3451 Views

Assessing the Toxoplasma Tachyzoite Cell Cycle Phases Using Fluorescent Ubiquitination-Based Cell Cycle Indicator
Mrinalini Batra and Elena S. Suvorova
Jan 20, 2026 109 Views
Abstract
Babesiosis is a tick-borne disease caused by pathogens belonging to the genus Babesia. In humans, the disease presents as a malaria-like illness and can be fatal in immunocompromised and elderly people. In the past few years, human babesiosis has been a rising concern worldwide. The disease is transmitted through tick bite, blood transfusion, and transplacentally in rare cases, with several species of Babesia causing human infection. Babesia microti, Babesia duncani, and Babesia divergens are of particular interest because of their important health impact and amenability to research inquiries. B. microti, the most commonly reported Babesia pathogen infecting humans, can be propagated in immunocompetent and immunocompromised mice but so far has not been successfully continuously propagated in vitro in human red blood cells (hRBCs). Conversely, B. divergens can be propagated in vitro in hRBCs but lacks a mouse model to study its virulence. Recent studies have highlighted the uniqueness of B. duncani as an ideal model organism to study intraerythrocytic parasitism in vitro and in vivo. An optimized B. duncani in culture and in mouse (ICIM) model has recently been described, combining long-term continuous in vitro culture of the parasite in human red blood cells with an animal model of parasitemia (P) and lethal infection in C3H/HeJ mice. Here, we provide a detailed protocol for the use of the B. duncani ICIM model in research. This model provides a unique and sound foundation to gain further insights into the biology, pathogenesis, and virulence of Babesia and other intraerythrocytic parasites, and has been validated as an efficient system to evaluate novel strategies for the treatment of human babesiosis and possibly other parasitic diseases.
Graphical abstract:
ICIM model [Adapted and modified from Pal et al. (2022)]
Background
Babesiosis is an emerging tick-borne disease caused by apicomplexan parasites of the genus Babesia. Like the other apicomplexan parasite Plasmodium falciparum, the causative agent of human malaria, Babesia parasites also invade human red blood cells (hRBCs) to cause the pathological symptoms associated with human babesiosis, with clinical outcomes ranging from mild to severe and, in some cases, leading to death. More than 100 species of Babesia are known to cause infection in a wide range of mammalian hosts, including livestock, with significant health and economic impacts (Renard and Ben Mamoun, 2021). Human babesiosis is an emerging worldwide concern; although humans are not the natural host of Babesia. Over the past 50 years there has been a rapid increase in cases of human babesiosis caused by different Babesia species (Renard and Ben Mamoun, 2021). While B. microti is the most common species causing human babesiosis, other species such as B. divergens and B. duncani have also been shown to lead to severe and sometimes lethal clinical outcomes (Persing et al., 1995; Rożej-Bielicka et al., 2015; Vannier et al., 2015; Kumar et al., 2021; Renard and Ben Mamoun, 2021). The first case of human babesiosis was identified in a splenectomized patient in Europe, but most cases of babesiosis found in northeastern and midwestern United States had no history of immune impairment (Skrabalo and Deanovic, 1957; Hunfeld et al., 2008; Vannier et al., 2015). Although humans are a dead-end host of Babesia parasites, cases of accidental transmission through blood transfusion from Babesia-infected individuals have been reported, and some rare cases of transplacental transmission have also been documented (Fox et al., 2006, Walker et al., 2022). The first case of transfusion-transmitted babesiosis (TTB) was reported in 1979, ten years after the first reported clinical case of B. microti human babesiosis in the United States (Scholtens et al., 1968, Jacoby et al., 1980). Although B. microti is the most common agent of TTB, cases caused by B. duncani have also been reported (Kjemtrup and Conrad, 2000; Kjemtrup et al., 2002). As the recipients are often immunocompromised, TTB could be fatal (Herwaldt et al., 1997; Claycomb et al., 1998; Conrad, 2000; Leiby, 2011; Renard and Ben Mamoun, 2021). The blood donors carrying Babesia parasites are often asymptomatic, which highlights the necessity for generating tools for efficient diagnosis of parasite infection and for the development of a vaccine and new therapies for the prevention and treatment of human babesiosis. For a long time, the life cycle and biology of Babesia parasites have been poorly elucidated, mainly due to the lack of suitable tools for continuous in vitro propagation of the parasites and/or lack of animal models to study their pathogenesis and virulence. The in culture and in mouse (ICIM) model for Babesia infection described herein will assist in answering some key questions about Babesia biology, pathogenesis, and survival in human red blood cells, namely how these parasites interact with the host and modulate its immune response. The ICIM model has also been important in the discovery and development of novel antibabesial drugs (Lawres et al., 2016; Chiu et al., 2021; Pal et al., 2022). In previous studies (Abraham et al., 2018), the continuous in vitro culture of B. duncani in hRBCs was reported in commercially available Claycomb (Sigma) and HL1 (Lonza) media. The HL1 medium has been discontinued since March 2021; the Claycomb medium often suffers supply shortages, is relatively expensive, and contains several mammalian proteins (bovine albumin, fetuin, transferrin, human insulin, long R3IGF-1, and long EGF) that, while important for HL-1 cardiomyocytes, are of no importance to Babesia intraerythrocytic development (Claycomb et al., 1998; White et al., 2004; Singh et al., 2022). We have recently reported that the DMEM/F-12 is an alternative growth medium for continuous in vitro culture of B. duncani in hRBCs (Singh et al., 2022). It differs from a standard DMEM medium, which does not support the growth of the parasite, by the presence of six amino acids (alanine, asparagine, aspartic acid, cysteine, glutamic acid, and proline), two vitamins (biotin and cobalamin), and four inorganic salts (cupric sulfate, ferric sulfate, magnesium chloride, and zinc sulfate) as well as hypoxanthine, thymidine, linoleic acid, lipoic acid, and putrescine (Singh et al., 2022). Furthermore, an optimized animal model of B. duncani infection that allows a consistent and reproducible evaluation of parasite development and virulence in mice has been recently described (Pal et al., 2022). The ICIM model, which combines the in vitro propagation of the parasite in human red blood cells and parasite virulence in mice, will usher a new era of advanced research on Babesia by facilitating the use of genetic tools and resources to conduct large scale functional analysis to link gene expression and function to disease progression and parasite virulence. This model will greatly enhance our understanding of this disease, as well as help in developing novel therapeutic strategies with improved efficacy.
Materials and Reagents
For in vitro culture
6-well plate (Corning, catalog number: 353046)
1.7 mL microcentrifuge tubes (Thomas Scientific, catalog number: 1149K01)
Centrifuge tubes (Corning, Falcon tubes, catalog numbers: 430829 [50 mL], 352096 [15 mL])
Pipette tips [USA Scientific, catalog numbers: 1121-3810 (10 μL), 1120-8810 (200 μL), 1111-2721 (1,000 μL)]
Plugged serological sterile pipettes [Corning, Falcon pipettes, catalog numbers: 357543 (5 mL), 357551 (10 mL), 357525 (25 mL)]
Millex filter units [Millipore, catalog numbers: SLGSR33SS (Syringe driven), S2GPU10RE (vacuum driven)]
Pipettes [Eppendorf, catalog numbers: K24694J (1,000 μL), J46084J (200 μL), H16607J (20 μL), I54818J (10 μL)]
Pipette AID (Drummond Scientific)
Aspiration pipettes (Santa Cruz, catalog number: 357781)
DMEM/F-12 (Lonza, catalog number: BE04-687/U1, Basel, Switzerland; or Thermo Fisher Scientific, catalog number: 21331020)
DMEM (Thermo Fisher Scientific, Gibco, catalog number: 11-966-025)
RPMI (Thermo Fisher Scientific, Gibco, catalog number: 11-875-093)
FBS (Thermo Fisher Scientific, Gibco, catalog number: 10438-026, Waltham, MA, USA)
50× HT media supplement Hybrid-MaxTM (Sigma, catalog number: H0137)
L-glutamine (Thermo Fisher Scientific, Gibco, catalog number: 25030-081)
Antimycotic (antibiotic) (Thermo Fisher Scientific, Gibco, catalog number: 15240-062)
Gentamicin reagent solution (Thermo Fisher Scientific, Gibco, catalog number: 15710-072)
A+ human RBCs (American Red Cross or Interstate Blood Bank, Inc.)
B. duncani WA1 strain (BEI Resources, NR-12311)
DMEM/F-12 complete media (250 mL) (see Recipes)
For cryopreservation
Glycerolyte 57 solution (Baxter Healthcare corporation, Deerfield, IL, USA, catalog number: 4A7831)
Cryotube vials (Thermo Scientific, catalog number: 363401)
For slide preparation
Premium microscope slides (Fisherfinest, catalog number: 22038-103)
Hemacolor solution I, fixative (Sigma-Aldrich, catalog number: 65044A-85)
Hemacolor solution II (Sigma-Aldrich, catalog number: 65044B-85)
Hemacolor solution III (Sigma-Aldrich, catalog number: 65044C)
Immersion oil (Cargille, catalog number: 16482)
For mouse infection
BD PrecisionGlideTM 27G needle (BD, catalog number: 305109)
Lithium heparin–coated blood collection tube (McKesson 574507, Greiner Bio-One, MiniCollect, catalog number: 450477)
Heparinized capillary tubes (Fisher Scientific, catalog number: 22-260-950)
Isoflurane (Covetrus, catalog number: 11695-6777-2)
PEG 400 (Thermo Fisher Scientific, Avantor J.T. Baker U216-07, catalog number: 02-003-646)
1× PBS diluted from 10× PBS pH 7.4 (Gibco, catalog number: 70011-044)
Mouse strains: C3H/HeJ (from Jackson Laboratories, Bar Harbor, ME)
Note: Our studies have shown that C3H/HeJ mice are susceptible to B. duncani infection following IV inoculation with doses of infected RBCs (iRBCs) ranging between 102 and 107. We found that 107 and 106 iRBC doses elicit an acute increase in parasitemia (P) (up to 35%) within a short span of time [3–5 days post infection (DPI)], whereas mice inoculated with doses of 102–105 iRBCs show a delayed onset of infection with a lower peak P (Pal et al., 2022). Parasitemia levels in C3H/HeJ mice were always higher in females compared to males at the same infection dose (Aguilar-Delfin et al., 2001, Pal et al., 2022). In contrast, C57BL/6J mice showed 100% survival with no detectable P following inoculation with 104 B. duncani–iRBC. Infection of Balb/cJ with 104 B. duncani–iRBC resulted in increased P over time in all mice; however, approximately 50% of the mice cleared the infection and survived, whereas the other half continued to show detectable P and succumbed to infection (Pal et al., 2022).
30% isoflurane (50 mL) (inhalation anesthetic; see Recipes)
Equipment
Sorvall legend XTR centrifuge (Thermo Scientific, catalog number: 75004521)
Microscope (Nikon Eclipse 5Oi)
SterilGARD III advance biological safety cabinet (The Baker Company, catalog number: SG603)
Water bath (Fisher brand, model: FSGPD15D)
Tri gas incubator (Thermo Scientific, model: HERACELL VIOS 160i)
Eppendorf MiniSpin (Millipore-Sigma, catalog number: EP022620100)
Software
GraphPad Prism version 9.4.1
Procedure
In vitro culture of B. duncani WA1 isolate in hRBCs
Prepare media by adding all the components (see Figure 1) in sterile conditions inside a biosafety cabinet (Figure 2).
Note: In specific circumstances when DMEM/F-12 is not available from the different mentioned sources, DMEM base medium supplemented with missing components (those present in DMEM/F-12) could be successfully used for parasite culture (see Table 1 provided below).
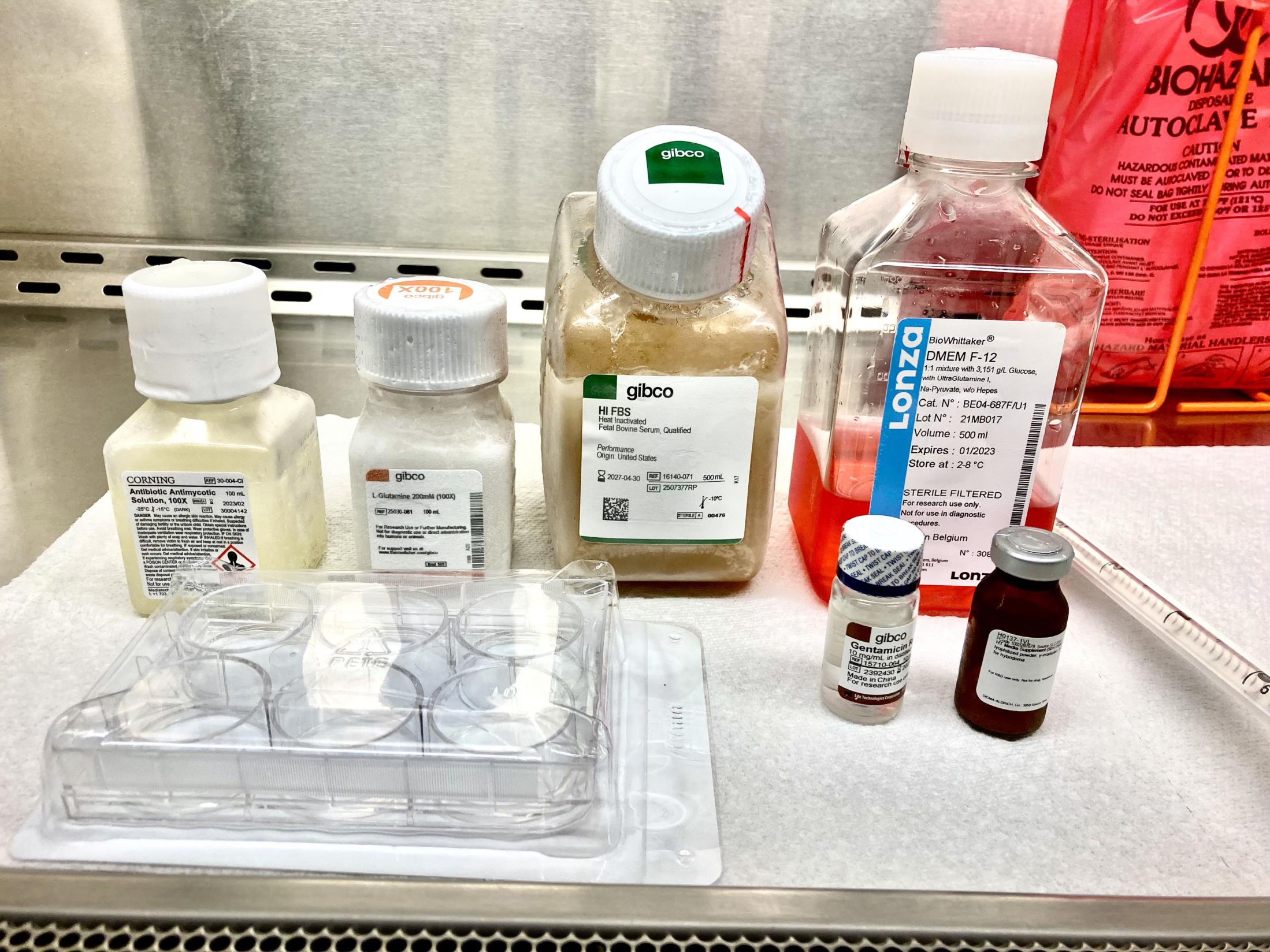
Figure 1. Representative image showing media components and materials required for B. duncani in vitro culture
Figure 2. Representative image showing biosafety cabinet BSL-2 grade required for handling and culturing of B. duncani parasiteTable 1. Supplement required to complete DMEM base medium to be used as a replacement of DMEM/F-12
Catalog number DMEMb
Catalog number: 11965092 (Thermo Fisher Scientific)
Conc. (mg/L)DMEM/F-12
Catalog number: BE04-687F/U1 (LONZA)
Conc. (mg/L)Amino Acids L-Alanine A7219, Sigma - 4.45 L-Asparagine-H2O A7094, Sigma - 7.5 L-Aspartic acid A7219, Sigma - 6.65 L-Cysteine hydrochloride-H2O C6852, Sigma - 17.56 L-Glutamic Acid G8415, Sigma - 7.35 L-Proline P5067, Sigma - 17.27 Vitamins Biotin B4639, Sigma - 0.004 Vitamin B12 V6629, Sigma - 0.68 Inorganic Salts Cupric sulfate (CuSO4·5H2O) C8027, Sigma - 0.0012 Ferric sulfate (FeSO4·7H2O) F8633, Sigma - 0.42 Magnesium Chloride (MgCl2) anhydrous M8266, Sigma - 28.57 Zinc sulfate (ZnSO4·7H2O) Z0251, Sigma - 0.43 Lipids Linoleic Acid L1012, Sigma - 0.044 Lipoic Acid T1395, Sigma - 0.013 Other components Putrescine 2HCl P5780, Sigma - 0.081 Hypoxanthine and Thymidine mix H0137-10VL, Sigma - 0.0112 - 0.001468 Wash hRBCs as follows:
After receiving blood (bag containing approximately 500 mL of total blood) from a donation center, mix gently by inverting the bag 2–3 times. Aliquot blood samples into 50 mL centrifuge tubes and store at 4 °C until washing. Unwashed blood can be stored at 4 °C for up to one month.
To wash an aliquot of blood, use incomplete DMEM or RPMI media. Use roughly four volumes of medium per one volume of packed RBCs. Mix the suspension gently to resuspend the RBCs and centrifuge at 1,800 rpm (757 × g) for 10 min at room temperature (RT).
Note: For washing RBCs, DMEM/RPMI from any source can be used.
Following centrifugation, gently aspirate the medium and carefully remove the buffy coat containing the white blood cells from the top RBC layer. Repeat the washing steps twice.
After the final wash, add an equal volume of incomplete DMEM or RPMI media to make 50% hematocrit (HC).
The washed RBCs (50% HC) can be immediately used for in vitro culture or stored at 4 °C for further use. Washed RBCs can be stored at 4 °C and used for up to two weeks in cell culture.
Thawing cryo-preserved B. duncani–infected erythrocytes:
Take a cryovial of frozen parasite from the liquid nitrogen and immediately put in a 37 °C water bath for a few seconds until the contents of the vial turn into liquid.
Transfer the contents of the thawed vial containing the B. duncani–infected hRBCs to a 50 mL centrifuge tube inside the biosafety cabinet.
Add 200 μL of 12% (w/v) NaCl (0.2× original cryovial volume) dropwise while gently shaking the tube. Incubate for 5 min at RT.
Add 9 mL of 1.6% (w/v) NaCl dropwise while gently shaking the tube.
Centrifuge at 1,500 rpm (526 × g) for 5 min at RT. Remove the supernatant without disturbing the pellet.
Add 25 mL of the incomplete DMEM/F-12 medium to wash the parasite pellet. Centrifuge at 1,500 rpm (526 × g) for 5 min at RT.
Repeat the above step one more time and resuspend the pellet in 3.6 mL of complete DMEM/F-12 media (see Recipes). Add 400 μL of washed 50% HC A+ RBCs (step 2) to maintain culture at 5% HC.
Seed the culture into a well of 6-well plate and place it in the incubator at 2% O2, 5% CO2, and 93% N2 atmosphere with 95% humidity setting (see Figure 3).

Figure 3. Representative image of Tri gas incubator used for B. duncani in vitro cultureMaintain the culture as follows:
Replace culture medium every day by aspirating old medium with aspirating pipette and replace with complete DMEM/F-12 medium prewarmed to 37 °C.
Note: Take extra precaution while aspirating the medium to not disturb the bottom of the plate containing the iRBCs.
Monitor the culture and P level by smear preparation as follows:
Aspirate medium as described above and resuspend culture in fresh medium by gently pipetting a few times.
Take 50 μL culture in a microcentrifuge tube and spin at 2,500 rpm (700 × g) for 2 min at RT.
Carefully remove supernatant with a 200 μL pipette tip (set to 40 μL), leaving behind 10 μL of medium. Resuspend RBCs pellet by gently pipetting a few times.
Place a drop of the resuspended RBCs on a slide and make a smear by sliding another slide at an angle of 25°–45° as shown in Figure 4.
Fix the blood smear for 10 s in the fixative solution I followed by 10 s in solution II. Stain with the solution III for 25 s. Rinse the stained slide in water, air dry, and visualize the slide under the light microscope at 100× (representative image of blood smear with 10% P shown in Figure 5).
Estimate P as follows:
Make a smear from in vitro culture and perform Giemsa staining as described above in steps i–v.
Air dry for 3–4 min. Put a drop of immersion oil and visualize under microscope.
Count iRBCs and total RBCs (tRBCs) (tRBCs = iRBCs + uninfected RBCs) in the field.
Repeat step 3 for different fields until the tRBCs count from adding all the fields is 1,000–2,000 RBCs (for more accuracy).
Calculate percentage of iRBCs as follows:
Field 1=(9 iRBCs)/(80 tRBCs) Field 2=(10 iRBCs)/(110 tRBCs) Field 3=(11 iRBCs)/(120 tRBCs) Field 5=(8 iRBCs)/(70 tRBCs) Field 6=(12 iRBCs)/(130 tRBCs)
(9+10+11+8+12)/(80+110+120+70+130)= 50/510×100=9.8%

Figure 4. Representative image demonstrating angular position of slide and RBC pellet (50 μL of in vitro culture after centrifugation) for smear preparation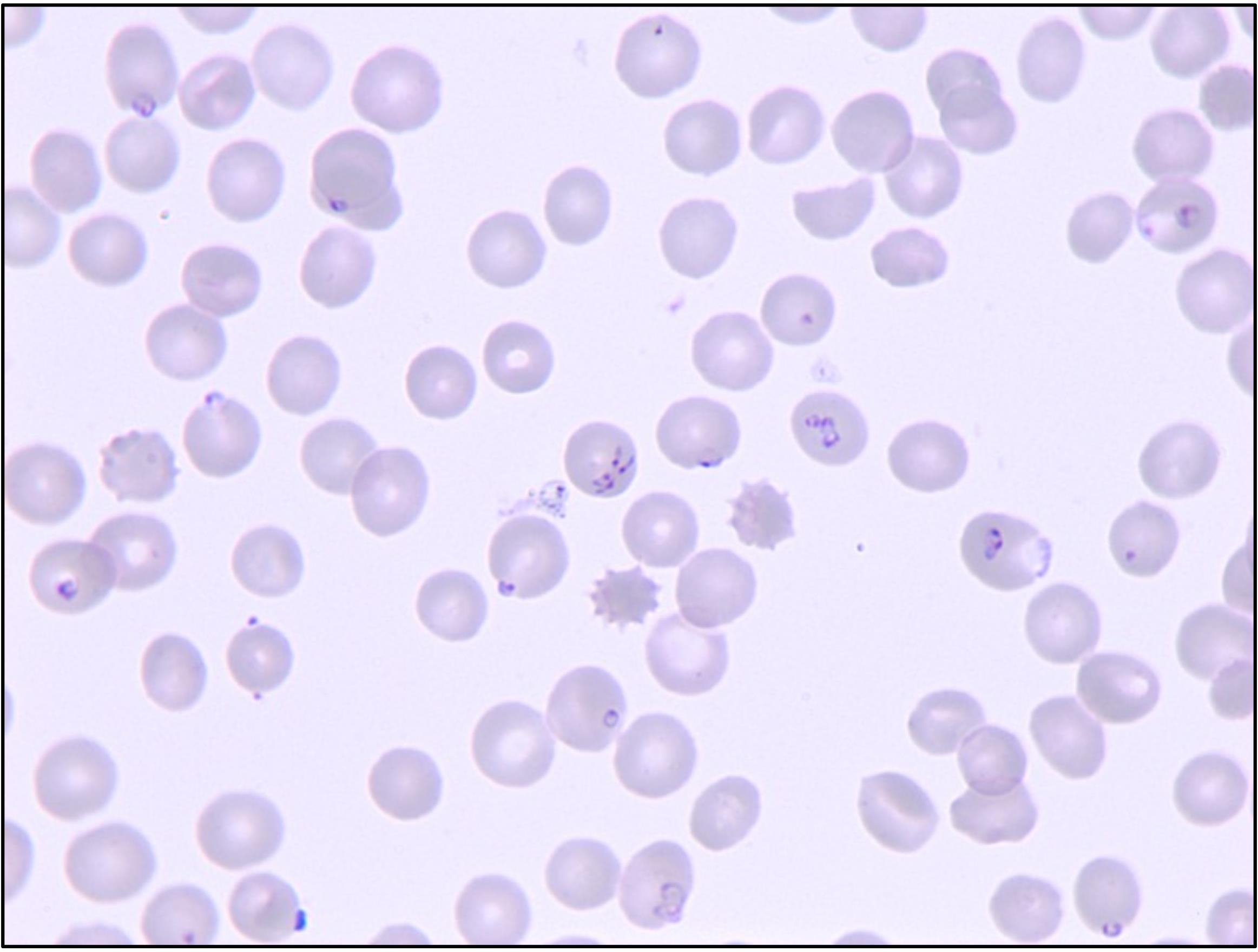
Figure 5. Representative image depicting 10% P of B. duncani–infected human erythrocytesNote: Increasing the number of fields for calculating the proportion of iRBCs and tRBCs and percentage of P levels allows a more accurate determination of P.
Maintain the parasite culture by inoculating 0.5%–1% P of original culture once the P level reaches 10%–15%.
Example: For a starter culture of 5 mL with 0.5% P and 5% HC, take 250 μL of the stock culture (10% P and 5% HC) and add 475 μL of 50% HC washed hRBCs and 4.275 mL of complete DMEM/F-12 medium.
For specific assays that require high P, culture the parasites continuously with media replacement after every 24 h until it reaches the desired P. B. duncani in vitro cultures can reach up to 20%–25% P.
Cryopreservation of in vitro cultured B. duncani–iRBCs
Estimate P levels by light microscopy following Giemsa staining of blood smears prepared from in vitro cultured parasites as described in step A4a if P is >5%, then proceed with the following steps of cryopreservation.
Pellet the culture (5 mL) by centrifugation at 1,800 rpm for 5 min at RT.
Aspirate the supernatant leaving behind the RBC pellet.
Add 250 μL of filter-sterilized glycerolyte dropwise while tapping the bottom of the tube to mix.
Gently mix the suspension and put in a cryovial. Label the cryovial describing the parasite strain and % P of the culture on the day of cryopreservation.
Store the cryovial at -80 °C.
For long-term storage (more than six months), take out the cryovial from -80 °C and place in liquid nitrogen.
Purification of B. duncani merozoites
Initiate and maintain B. duncani in vitro culture in hRBCs in DMEM/F-12 medium until P reaches 18%–20% and free merozoite can be observed in blood smears (Figure 6).
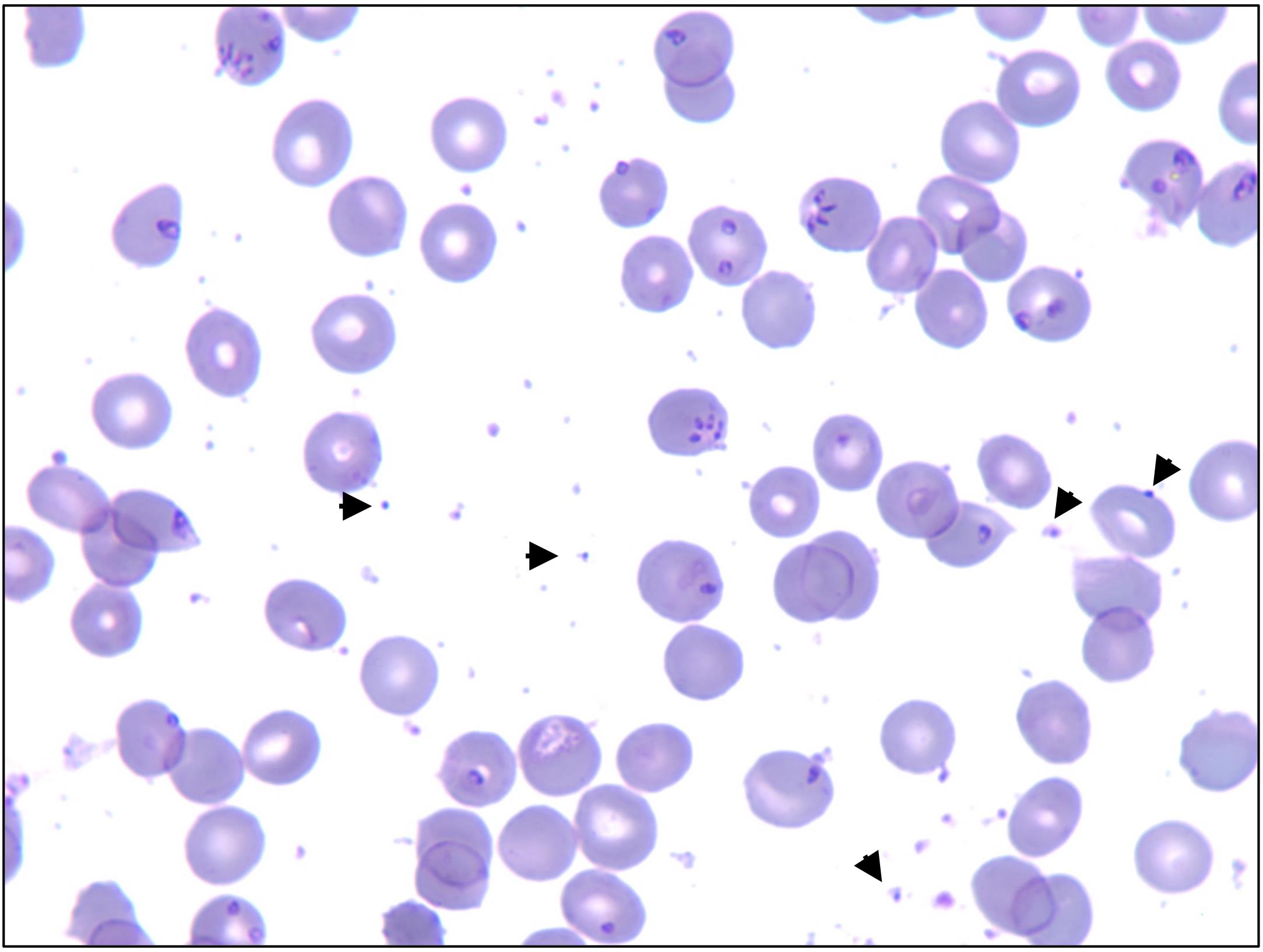
Figure 6. Representative image depicting 18%–20% P with free merozoites indicated by arrowsTo isolate the free merozoites, centrifuge the parasite culture at 1,800 rpm (757 × g) and 37 °C for 5 min.
Use the pellet containing the B. duncani iRBCs for initiating fresh starter cultures or for other assays. The supernatant containing the free merozoites is collected using a pipette in a fresh centrifuge tube.
The supernatant is centrifuged at 4,000 rpm (1,932 × g) and 37 °C for 10 min. The resulting pellet contains the free merozoites.
Resuspend the merozoite pellet in warm DMEM/F-12 in 1:5 ratio (v/v). For example, for a packed pellet of 10 μL, add 50 μL of culture medium.
Estimate the purity of the merozoites by Giemsa staining. The purity of a merozoite preparation is determined by the amount of free merozoites with little to no intact uninfected or infected RBCs. Figure 7 shows an example of a merozoite preparation with no RBCs detected. The merozoite preparations that are more than 95% pure are considered suitable for subsequent cell biological and molecular analyses.
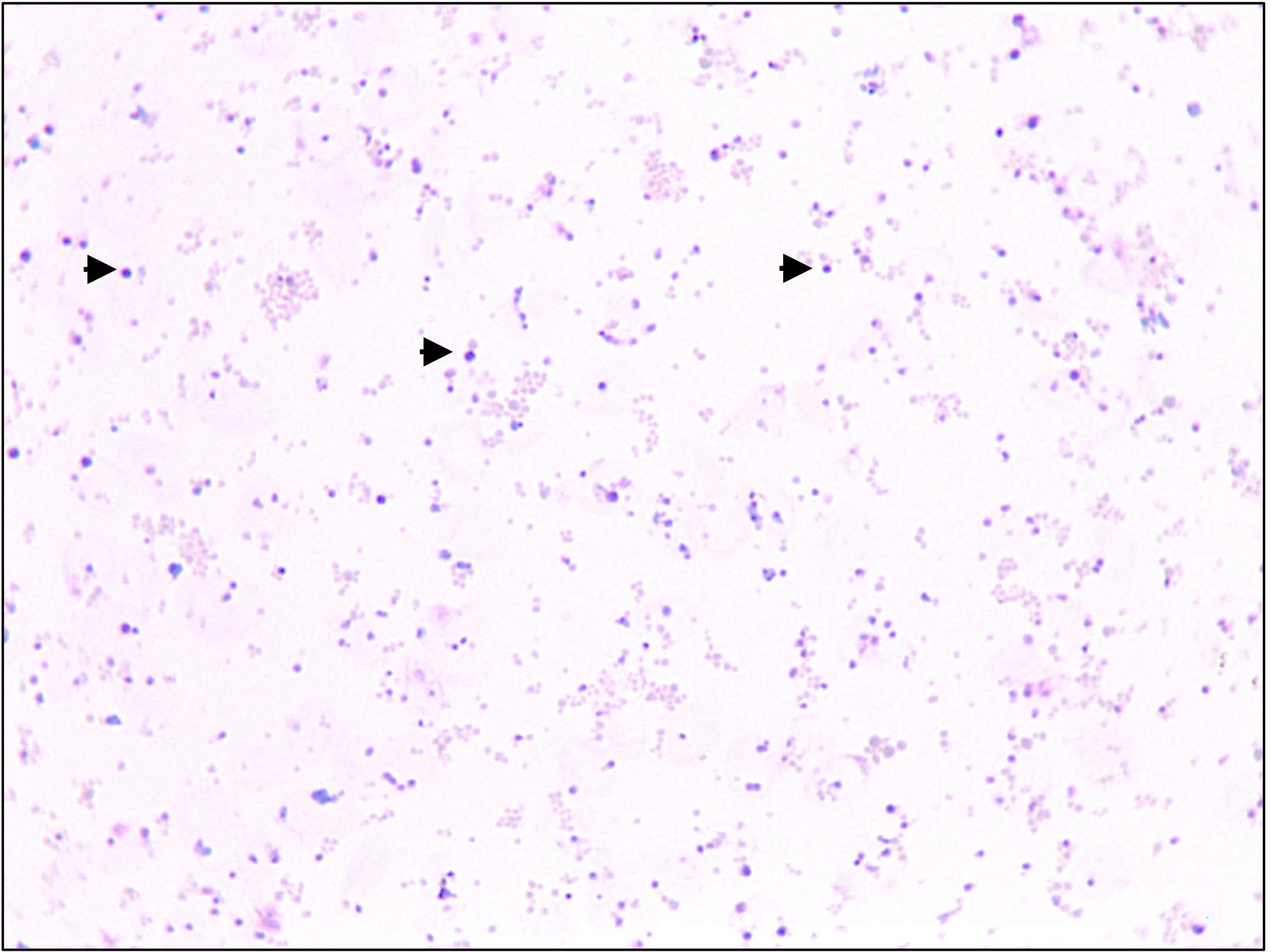
Figure 7. Representative image showing free merozoites (black arrows). Pink structures in the image represent RBC membranes resulting from lysis of infected erythrocytes.Mouse infection
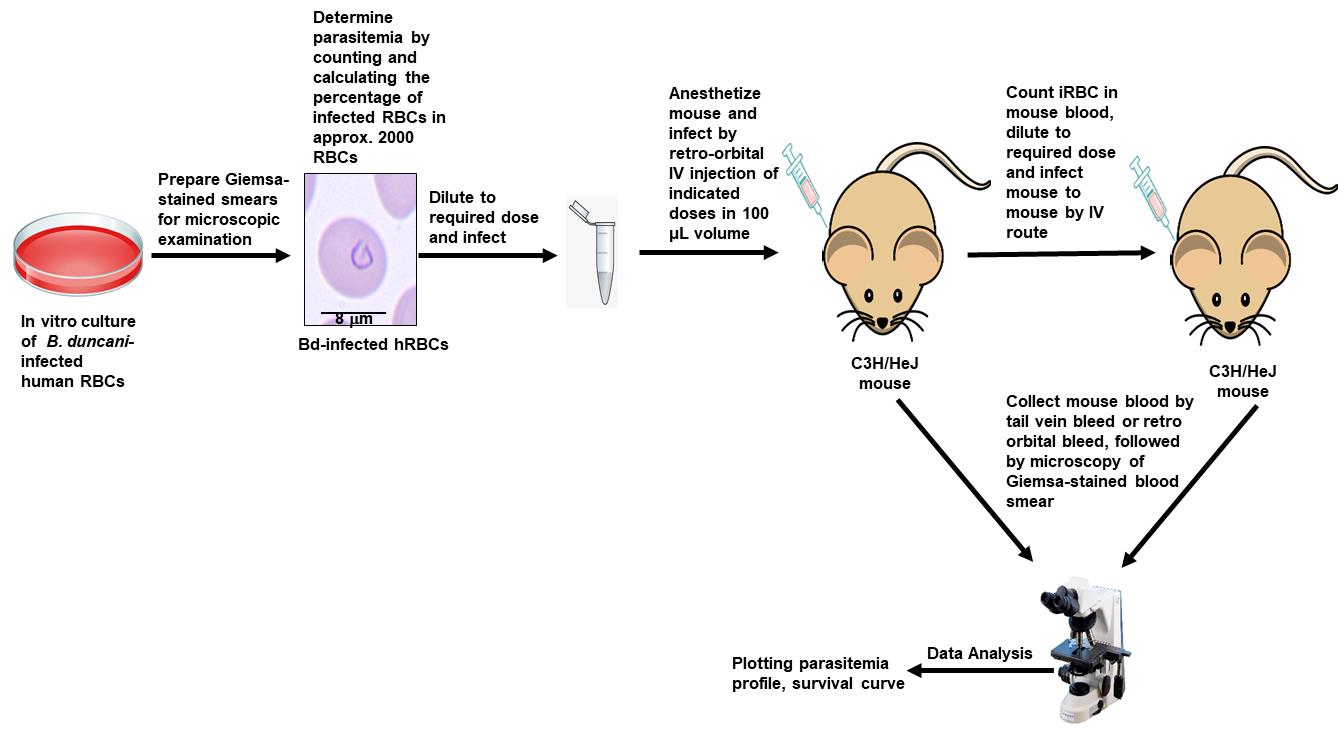
Use 5–6 weeks old female C3H/HeJ mice after one week acclimatization post procurement.
To infect mouse, use either B. duncani in vitro cultured purified merozoites (2 × 107) or human iRBCs (8.5 × 105) and inject by retro-orbital intravenous (IV) route.
Note: Higher doses of parasite infection will expedite the establishment of infection in mouse. However, results may vary depending on the success of infection and handling.
Calculate the amount of ihRBCs containing 8.5 × 105 parasites as follows:
Calculation of iRBCs in the in vitro culture:
1 mL of human blood = 5 × 109 RBCs (100% HC)
1 mL of 5% HC human blood = 2.5 × 108 RBCs
If the P estimated by Giemsa stain is 10%
1 mL of 5% HC with 10% P = 10/100 × 2.5 × 108 = 2.5 × 107 iRBCs
Take the desired amount of parasite (8.5 × 105) by serial dilution as follows:
Take 100 μL of above culture (step D3a) containing 2.5 × 106 iRBCs and dilute 1:10 by adding 900 μL of 1× PBS to attain a concentration of 2.5 × 103 parasites per μL.
For 8.5 × 105 parasites from above diluted culture, take 340 μL (8.5 × 105 ÷ 2.5 × 105 = 340 μL) and pellet down by centrifugation at 1,800 rpm (757 × g) for 5 min at RT. Carefully aspirate the supernatant leaving behind 100 μL of supernatant.
Resuspend by gentle tapping and inject as described below in step D5.
Note: A serial dilution to take the desired amount of parasite is important, rather than taking a very little amount (e.g., 34 μL) directly from original culture (to avoid pipetting error).
Perform IV infection as follows:
Use a jar with cotton soaked in 30% isoflurane (inhalation anesthetic) diluted in PEG 400 placed at the bottom and covered with a mesh. Place a single mouse inside the sealed chamber containing the above-mentioned inhalation anesthetic (see Figure 8).

Figure 8. Representative image showing reagents and materials required for mouse infectionInject 100 μL of parasite (in PBS) diluted as mentioned above in step D3b and inject via IV route.
Monitor P by microscopy of thin blood smears prepared from blood collected from the tail vein on a regular interval of 24 or 48 h. Plot the P and survival curve using GraphPad Prism.
Blood from an infected mouse can be used to infect another uninfected mouse to maintain the parasite stock. Parasitemia in the stock mouse can be monitored by microscopic examination of Giemsa-stained blood smears.
Calculate the amount of blood required for mouse-to-mouse infection:
Calculation of iRBCs
1 mL of mouse blood = 10 × 109 RBCs
If P (estimated by Giemsa stain) is 10%
1 mL of 10% P = 10/100 × 10 × 109 = 109 iRBCs
Dilution of iRBCs
If the desired dose of infection is 104 parasites, collect 30 μL of blood from the mouse by retroorbital bleeding in a heparinized blood collection tube and dilute as follows:
Take 10 μL of blood (1.0 × 107 iRBCs) and add 990 μL of 1× PBS to make 1 × 107 iRBC/mL.
Dilute above diluted iRBCs to 1:10 for two more times by taking 100 μL each time from previous dilution and adding 900 μL of 1× PBS (serial dilution).
This will result in 1 mL containing 1 ×
105 iRBCs (102 iRBCs/μL) at final step. Use 100 μL (1 × 104 iRBC) of this dilution to inject each mouse.
Notes:
1. Calculate the dose for N+2 mice to account for any volume loss. Infection from mouse to mouse is established faster than from in vitro culture to mouse (see Pal et al., 2022).
2. A dose response infection (ranging from 102–107 iRBCs) was tested in mouse-to-mouse transmission of B. duncani and successful infection was achieved at all doses (Pal et al., 2022). Compared to higher doses, subsequent lower doses led to delays in the establishment of infection. However, results may vary depending on the handling.
Any animal showing signs of distress should be humanely euthanized per approved IACUC protocol by CO2 asphyxiation followed by cervical dislocation.
Data analysis
For in vitro studies (Figure 9), percent P on the indicated days (days 0, 3, 6, 9, 12, and 15) is determined by counting the number of iRBCs out of approximately 3,000 tRBCs per blood smear prepared from cultures grown in different media, as described in step A4a. The values are then plotted using GraphPad prism. Statistical significance (P-value) is calculated using two-way ANOVA.
For in vivo studies (Figure 10), P is estimated as described in step A4.a.vi from thin blood smears prepared from mouse blood at the indicated time points (DPI 1, 3, 4, 6, 7, 8, 9, 10, and 11). The number of iRBCs in a total of approximately 2,000 tRBCs is determined for each mouse. At least three mice are used per group. Data are then plotted using GraphPad prism 9.4.1 software. Kaplan-Meier survival curves are generated using GraphPad prism 9.4.1.
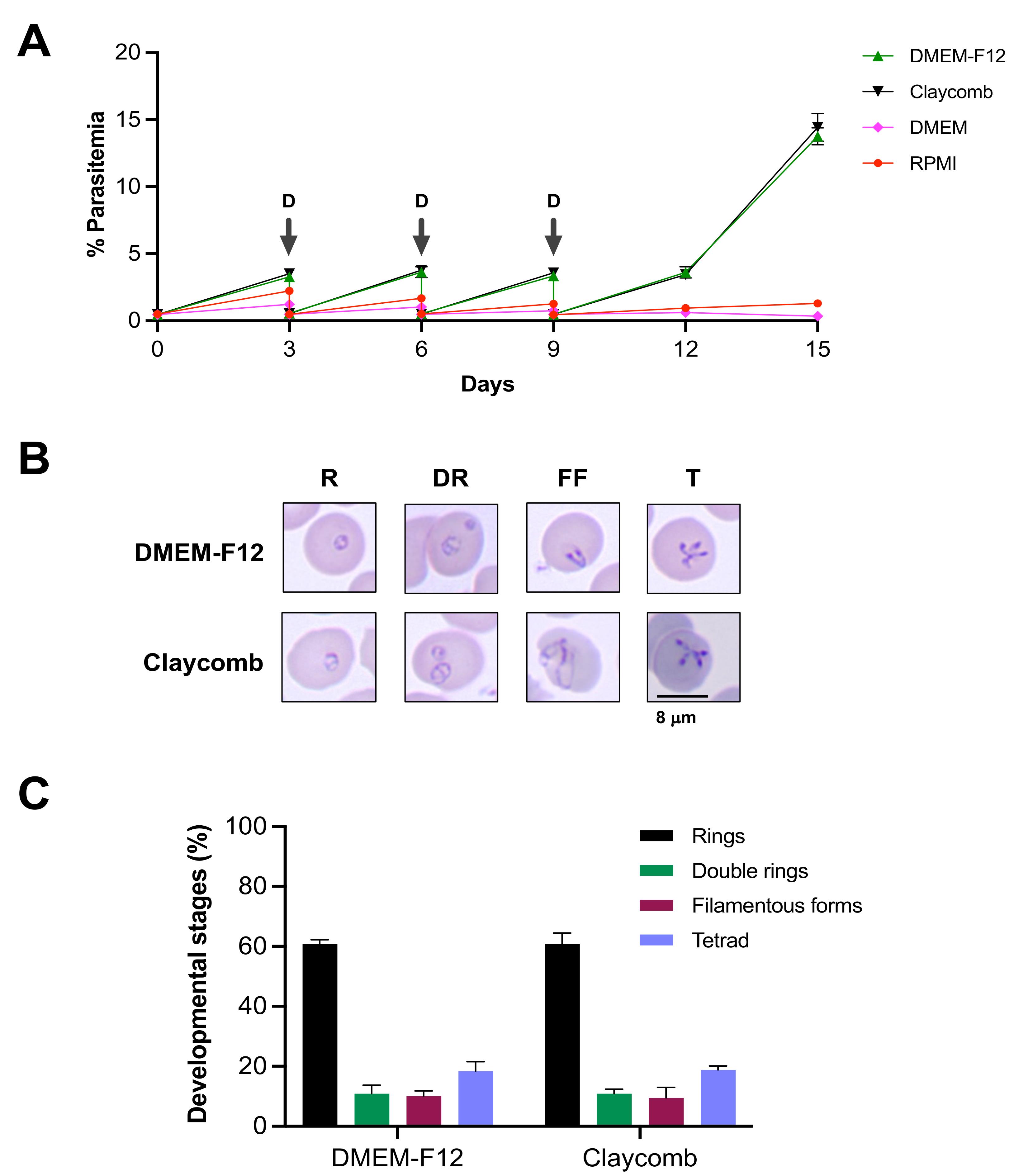
Figure 9. In vitro propagation of B. duncani WA1 in different growth media. (A) Continuous in vitro growth of B. duncani WA1 in different media for a period of 15 days in hRBCs. The cultures were diluted (D) on days 3, 6, and 9 (indicated by arrows). (B) Representative images of Giemsa-stained smears of in vitro cultured B. duncani WA1 parasites showing different stages, including rings (R), double rings (DR), filamentous forms (FF), and tetrads (T). (C) Graph showing percentage of different developmental stages of B. duncani WA1 in DMEM/F-12 and Claycomb media. Data presented are mean ± SD of two independent experiments performed in biological duplicates. No significant differences (p > 0.99, two-way ANOVA) were found between the different stages in the two media [adapted and modified from Singh et al. (2022)].
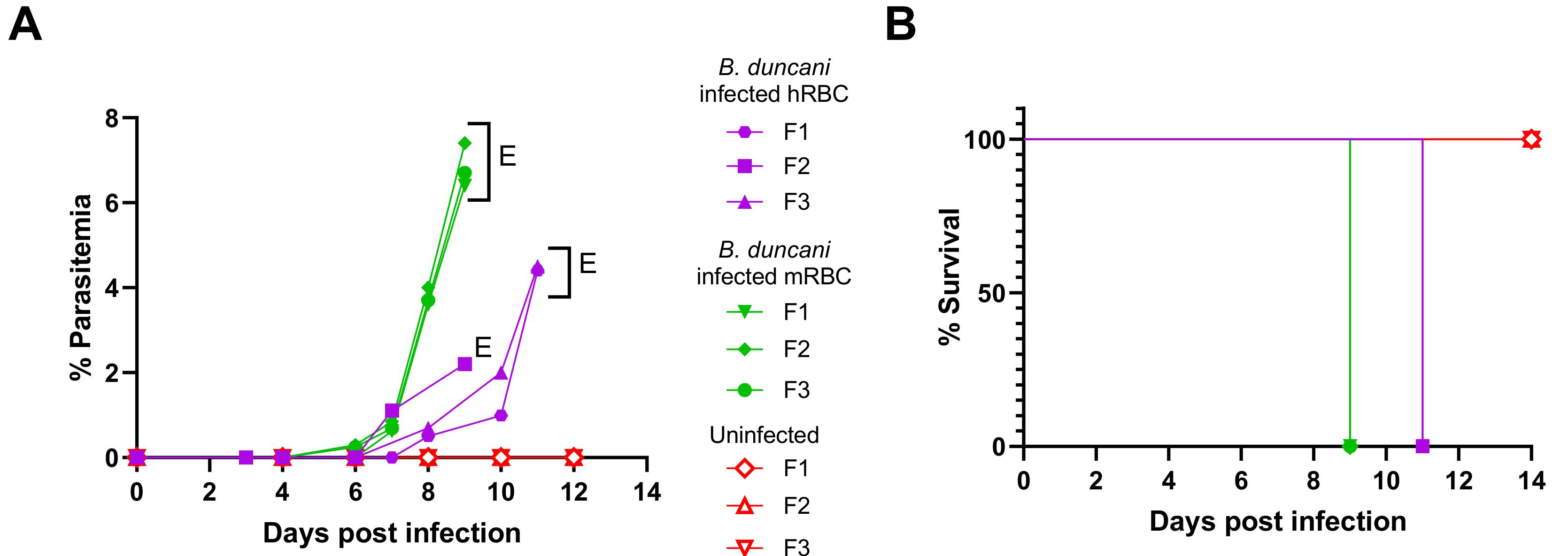
Figure 10. Lethal B. duncani infection in immunocompetent mice. (A) Parasitemia profile over time in female C3H/HeJ mice (n = 3/group) following infection with B. duncani parasites from in vitro culture in hRBCs (purple; 8.5 × 105 iRBC/mouse), or B. duncani parasitized mouse RBCs (mRBC) collected from infected mice (green; 1 × 104 iRBC/mouse). Uninfected mice profile depicted in red. E: euthanized. (B) Kaplan-Meier plot of percent survival of uninfected mice (red), mice infected with in vitro cultured B. duncani parasites (purple), or mice infected with B. duncani parasitized mouse blood (green) at indicated doses [adapted and modified from Pal et al. (2022)].
Recipes
DMEM/F-12 complete media (250 mL)
DMEM/F-12 188.5 mL
FBS 50 mL
HT media supplement (50×) hybrid max 5 mL
L-Glutamine 2.5 mL
Antimycotic 2.5 mL
Gentamycin 2.5 mL
30% isoflurane (50 mL)
Isoflurane 15 mL
PEG 400 35 mL
Acknowledgments
Funding: The published protocols by Pal and colleagues (Pal et al., 2022) and Singh and colleagues (Singh et al., 2022) were supported by the National Institutes of Health grants (AI123321, AI138139, AI152220, and AI136118), the Steven and Alexandra Cohen Foundation (Lyme 62 2020), and the Global Lyme Alliance to CBM.
Competing interests
None of the named authors have any conflict of interest, financial or otherwise.
Ethics
All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) at Yale University (Protocol #2020-07689). Animals were acclimatized for one week after arrival before the start of an experiment. Animals that showed signs of distress or appeared moribund were humanly euthanized using approved protocols.
Institutional Review Board Statement: All animal studies conducted were approved by the Institutional Animal Care and Use Committees (IACUC) at Yale University (Protocol #2020-07689).
Institutional Biosafety Statement: All studies involving the use of human blood and Babesia parasites in culture were approved by the Institutional BioSafety Committee at Yale University.
References
- Abraham, A., Brasov, I., Thekkiniath, J., Kilian, N., Lawres, L., Gao, R., DeBus, K., He, L., Yu, X., Zhu, G., Graham, M. M., Liu, X., Molestina, R. and Ben Mamoun, C. (2018). Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J Biol Chem 293(52): 19974-19981.
- Aguilar-Delfin, I., Homer, M. J., Wettstein, P. J. and Persing, D. H. (2001). Innate resistance to Babesia infection is influenced by genetic background and gender. Infect Immun 69(12): 7955-7958.
- Chiu, J. E., Renard, I., Pal, A. C., Singh, P., Vydyam, P., Thekkiniath, J., Kumar, M., Gihaz, S., Pou, S., Winter, R. W., et al. (2021). Effective Therapy Targeting Cytochrome bc1 Prevents Babesia Erythrocytic Development and Protects from Lethal Infection. Antimicrob Agents Chemother 65(9): e0066221.
- Claycomb, W. C., Lanson, N. A., Jr., Stallworth, B. S., Egeland, D. B., Delcarpio, J. B., Bahinski, A. and Izzo, N. J., Jr. (1998). HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95(6): 2979-2984.
- Fox, L. M., Wingerter, S., Ahmed, A., Arnold, A., Chou, J., Rhein, L. and Levy, O. (2006). Neonatal babesiosis: case report and review of the literature. Pediatr Infect Dis J 25(2): 169-173.
- Herwaldt, B. L., Kjemtrup, A. M., Conrad, P. A., Barnes, R. C., Wilson, M., McCarthy, M. G., Sayers, M. H. and Eberhard, M. L. (1997). Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J Infect Dis 175(5): 1259-1262.
- Hunfeld, K. P., Hildebrandt, A. and Gray, J. S. (2008). Babesiosis: recent insights into an ancient disease. Int J Parasitol 38(11):1219-37.
- Jacoby, G. A., Hunt, J. V., Kosinski, K. S., Demirjian, Z. N., Huggins, C., Etkind, P., Marcus, L. C. and Spielman, A. (1980). Treatment of transfusion-transmitted babesiosis by exchange transfusion. N Engl J Med 303(19): 1098-1100.
- Kjemtrup, A. M. and Conrad, P. A. (2000). Human babesiosis: an emerging tick-borne disease. Int J Parasitol 30(12-13): 1323-1337.
- Kjemtrup, A. M., Lee, B., Fritz, C. L., Evans, C., Chervenak, M. and Conrad, P. A. (2002). Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion 42(11): 1482-1487.
- Kumar, A., O'Bryan, J. and Krause, P. J. (2021). The Global Emergence of Human Babesiosis. Pathogens 10(11): 1447.
- Lawres, L. A., Garg, A., Kumar, V., Bruzual, I., Forquer, I. P., Renard, I., Virji, A. Z., Boulard, P., Rodriguez, E. X., Allen, A. J., et al. (2016). Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 213(7): 1307-1318.
- Leiby, D. A. (2011). Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin Microbiol Rev 24(1): 14-28.
- Pal, A. C., Renard, I., Singh, P., Vydyam, P., Chiu, J. E., Pou, S., Winter, R. W., Dodean, R., Frueh, L., Nilsen, A. C., et al. (2022). Babesia duncani as a Model Organism to Study the Development, Virulence, and Drug Susceptibility of Intraerythrocytic Parasites In Vitro and In Vivo. J Infect Dis 226(7):1267-1275.
- Persing, D. H., Herwaldt, B. L., Glaser, C., Lane, R. S., Thomford, J. W., Mathiesen, D., Krause, P. J., Phillip, D. F. and Conrad, P. A. (1995). Infection with a Babesia-like organism in northern California. N Engl J Med 332(5): 298-303.
- Renard, I. and Ben Mamoun, C. (2021). Treatment of Human Babesiosis: Then and Now. Pathogens 10(9): 1120.
- Rożej-Bielicka, W., Stypułkowska-Misiurewicz, H. and Gołąb, E. (2015). Human babesiosis. Przegl Epidemiol 69(3): 489-94, 605-8.
- Scholtens, R. G., Braff, E. H., Healey, G. A. and Gleason, N. (1968). A case of babesiosis in man in the United States. Am J Trop Med Hyg 17(6): 810-3.
- Singh, P., Pal, A. C. and Mamoun, C. B. (2022). An Alternative Culture Medium for Continuous In Vitro Propagation of the Human Pathogen Babesia duncani in Human Erythrocytes. Pathogens 11(5): 599.
- Skrabalo, Z. and Deanovic, Z. (1957). Piroplasmosis in man; report of a case. Doc Med Geogr Trop 9(1): 11-16.
- Vannier, E. G., Diuk-Wasser, M. A., Ben Mamoun, C. and Krause, P. J. (2015). Babesiosis. Infect Dis Clin North Am 29(2): 357-70.
- Walker, S., Coray, E., Ginsberg-Peltz, J. and Smith, L. (2022). A Five-Week-Old Twin With Profound Anemia: A Case Report of Asymmetric Congenital Babesiosis. Cureus 14(3): e22774.
- White, S. M., Constantin, P. E. and Claycomb, W. C. (2004). Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 286(3): H823-829.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kumari, V., Pal, A. C., Singh, P. and Ben Mamoun, C. (2022). Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy. Bio-protocol 12(22): e4549. DOI: 10.21769/BioProtoc.4549.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Microbiology > in vivo model > Protozoan
Cell Biology > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








