- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Step-by-step Protocol for Obtaining Mature Microglia from Mice
Published: Vol 12, Iss 15, Aug 5, 2022 DOI: 10.21769/BioProtoc.4481 Views: 2960
Reviewed by: Alessandro DidonnaYiqun YuThirupugal Govindarajan

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3913 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2411 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 213 Views
Abstract
In mice, microglial precursors in the yolk sac migrate to the brain parenchyma through the head neuroepithelial layer between embryonic days 8.5 (E8.5)–E16.5 and acquire their unique identity with a ramified form. Based on the microglial developmental process, we dissected the neuroepithelial layer (NEL) of E13.5 mice, which is composed of microglial progenitor and neuroepithelial cells. The NEL was bankable and expandable. In addition, microglial precursors were matured according to NEL culture duration. The matured microglia (MG; CD11b-positive cells) were easily isolated from the cultured NEL using a magnetic-activated cell sorting system and named NEL-MG. In conclusion, we obtained higher yields of adult-like microglia (mature microglia: NEL-MG) compared to previous in vitro surrogates such as neonatal microglia and microglial cell lines.
Graphical abstract:

Background
So far, microglial cell lines, primary fetal/neonatal microglia, and acute isolated adult microglia have been used in in vitro studies. However, low yields, immature phenotypes, and the use of many experimental animals are barriers to studying microglia. Here, we introduce a new method for obtaining bankable and expandable adult-like microglia. The neuroepithelial layer (NEL) of mice at embryonic day 13.5 (E13.5), which is composed of microglial progenitors and neuroepithelial cells, was dissected and then cultured or banked. Microglia (MG; CD11b-positive cells) were isolated from the cultured NEL using a magnetic-activated cell sorting system and named NEL-MG. This new method contributes to the obtainment of matured forms of microglia (adult-like microglia) with only a small number of experimental animals.
Materials and Reagents
Animals
Timed pregnant (13d; TP13) C57BL/6 mice (female, Daihan-Biolink Co., Chungbuk, Korea)
Culture products
Microtubes (Axygen, catalog number: MCT-150-C)
15 mL conical tubes (Thermo Fisher Scientific, catalog number: 339650)
100 mm dish (Thermo Fisher Scientific, catalog number: 150466)
T-25 Flasks (Thermo Fisher Scientific, catalog number: 156367)
Hanks’ balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14170-112)
75% ethyl alcohol anhydrous (Daejung Chemicals & Metals, catalog number: 4023-2304)
Trypsin 2.5% (Thermo Fisher Scientific, GibcoTM, catalog number: 15090-046)
Poly-D-lysine (PDL) (Sigma-Aldrich, catalog number: P7280-5MG)
Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11995-065)
Penicillin-Streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 16000-044)
GlutaMAXTM (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061)
Dulbecco’s phosphate buffered salt (DPBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144)
Trypan blue stain (0.4%) (Thermo Fisher Scientific, GibcoTM, catalog number: 15250-061)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650-5X5ML)
Bovine serum albumin fraction V (BSA) (Merck, catalog number: 10735086001)
CD11b (microglia) MicroBeads (Miltenyi Biotec, catalog number: 130-093-634)
MS columns (Miltenyi Biotec, catalog number: 130-042-201)
Culture medium (500 mL) (see Recipes)
70% ethanol (100 mL) (see Recipes)
0.25% trypsin (10 mL) (see Recipes)
PB buffer (50 mL) (see Recipes)
Equipment
Optical microscope (Olympus, model: SZ-ST)
Clean bench (LabTech, model: LCB-1201V)
Dissection tools
Mosquito forceps (KASCO, catalog number: S8-099)
Micro dissecting forceps (KASCO, catalog number: 50-2000-1)
Micro scissors (Medro Instruments, catalog number: 02-027-10)
Electronic forceps (KASCO, catalog number: 11-412-11)
Centrifuge machine (LaboGene, catalog number: 1248R)
Hemocytometer (Marienfeld superior, catalog number: HSU-0650030)
CO2 cell culture incubator (PHCbi, catalog number: MCO-18AC-PK)
37 °C water bath (DAIHAN Scientific, catalog number: DH.WHB00106)
Magnetic cell separator (Miltenyi Biotec, catalog number:130-042-102)
Software
ImageJ (National Institutes of Health, https://imagej.net)
Prism 7 (GraphPad, https://graphpad.com)
Biorender (Biorender, https://biorender.com)
Procedure
Mixed neuroepithelium–microglial progenitor cultures form a confluent layer and help microglial survival and yield. Processing steps for dissection and culture are as follows:
Note: This step should be performed on a clean bench.
Step 1: Isolation and culture of murine microglia
Coat a T-25 culture flask with 5 mL of PDL for 2 h in a humidified incubator (5% CO2, 37 °C). Wash the flask bottom with 5 mL of distilled water three times and dry before use.
Prepare the tools and reagents needed for the culture experiment. Spray the dissection tools and workspace with 70% ethanol. Warm up the culture medium in a 37 °C water bath.
Collect TP13 mice from the breeding cage. For each mouse, inject the pentobarbital at a dose of 0.03 mL using a syringe via the intramuscular (i.m.) route into the leg muscle (100 mg/kg in saline).
Using micro scissors, cut through the abdominal wall. Use micro dissecting forceps to lift up the uterine horns and cut away the uterus with scissors. Transfer the extracted uterus to ice-cold (4 °C) HBSS.
Cut the muscular uterine tissue with micro scissors in a 100 mm dish containing 20 mL of HBSS at room temperature (20–25 °C). Then, grab the muscular uterine tissue with both electronic forceps and pull away to take out the mouse embryos. Separate the embryos by cutting through the uterus in the regions between each embryo. The embryos may pop out spontaneously or come out after pressing gently with the forceps.
Add 20 mL of HBSS at room temperature in a separate 100 mm dish.
Transfer 6–10 embryos to the dish (Figure 1).
With one hand, pick up an embryo with the forceps. Using the other hand, cut off the head skin above the eyes (Figure 2, Video1).
Transfer the dissected tissue to a microtube. Add 1 mL of 0.25% trypsin and chop up the tissue into several pieces using micro scissors. Pipet up and down several times with 1 mL pipet, and then place the tube in the cell culture incubator at 37 °C for 3 min.
Transfer the cell suspension to a 15 mL conical tube. Add 10 mL of DPBS at room temperature and centrifuge the 15 mL conical tubes at 300 × g for 5 min at room temperature.
Aspirate the supernatant and resuspend the pellet with 5 mL of warmed culture media.
Calculate the cell density using a hemocytometer.
Seed the cell suspension into the coated bottom T-25 flask at a density of 200,000 cells/cm2. Add the culture media to reach a final volume of 7 mL in the flasks. Place the flasks into the cell culture incubator at 37 °C with 5% CO2.
Change the fresh culture medium the next day, and then every 2–3 days, to remove cell debris.
After 14–21days of seeding, check the density of cells in the T-25 flask (Figure 3). When the cells reach 90% confluence, split them into two flasks.
Note: If the mixed cells reach confluency, but microglia are not needed immediately, the mixed culture can be stored. Mixed cultures can also be frozen for a long time in freezing media composed of DMEM with 20% FBS and 10% DMSO in liquid nitrogen (about -196 °C).
When thawing the mixed cells, dissolve the stock vials in a water bath at 37 °C for 3 min and dilute with culture medium.
Step 2: Purification of murine microglia from the NEL culture
To collect microglia, add 1 mL of 0.25% trypsin and incubate for 3 min.
Tap the T-25 flasks and collect the floating cells in conditioned culture media in the 15 mL conical tubes.
Add 10 mL of DPBS and centrifuge the tubes at 300 × g for 5 min. Aspirate the supernatant completely.
Resuspend the cell pellet in 90 µL of cold PB buffer per 107 total cells by pipetting up and down.
Add 10 µL of CD11b (microglia) MicroBeads.
Mix well and incubate for 15 min in the dark at 4 °C.
Wash the cells by adding 1 mL of cold PB buffer per 107 cells and centrifuge them at 300 × g for 5 min. Aspirate the supernatant completely.
Resuspend up to 107 cells in 1,500 µL of PB buffer.
Place a column in the magnetic field of a suitable magnetic-activated cell sorting separator (MACS). Prepare the column by rinsing it with 500 µL of PB buffer.
Apply the cell suspension onto the column. Collect the flow-through containing unlabeled cells and perform passing steps by adding the buffer three times, each time after the column reservoir is empty (MS column: 500 µL × 3).
Pipette 1 mL of PB buffer onto the column. Immediately flush out the magnetically labeled cells by firmly pushing the plunger into the column. This is the target cell (microglia) fraction (Figure 4).
Note: To increase the purity of microglia, it is recommended to enrich the positive fraction over a second MS column. Repeat the magnetic separation procedure as described in steps B9 to B11 by using a new column.
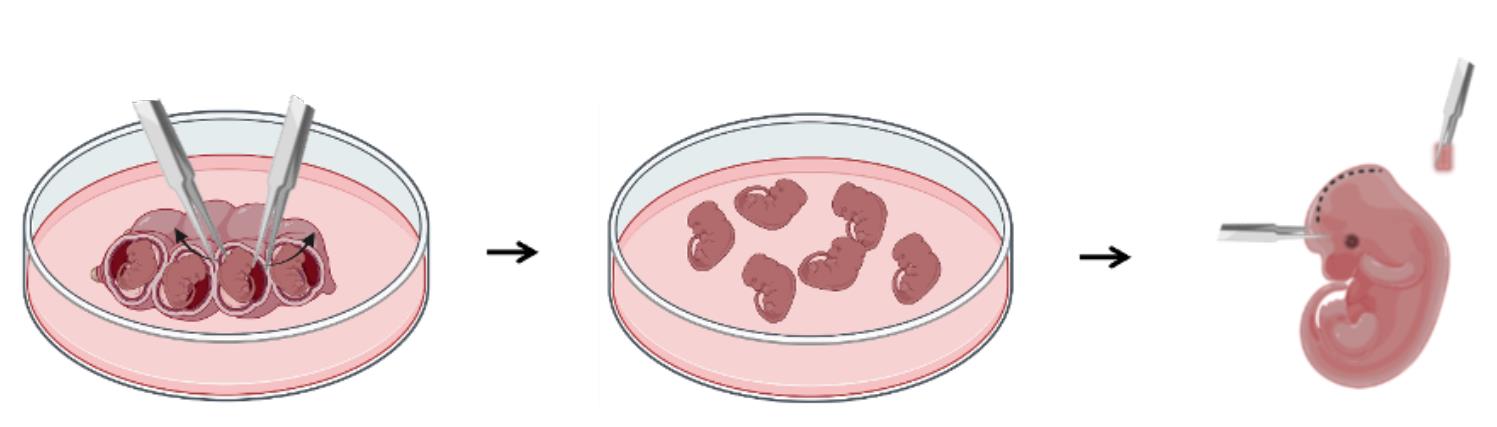
Figure 1. Processing of mouse embryos for neuroepithelial layer (NEL) culture. Firstly, separate the mouse embryos by cutting the uterus tissue in the regions between each embryo. Secondly, transfer the embryos to a new dish with cold HBBS—use one dish of fresh HBBS for every 6–10 embryos. Thirdly, use the micro dissecting forceps to dissect the head skin of embryos.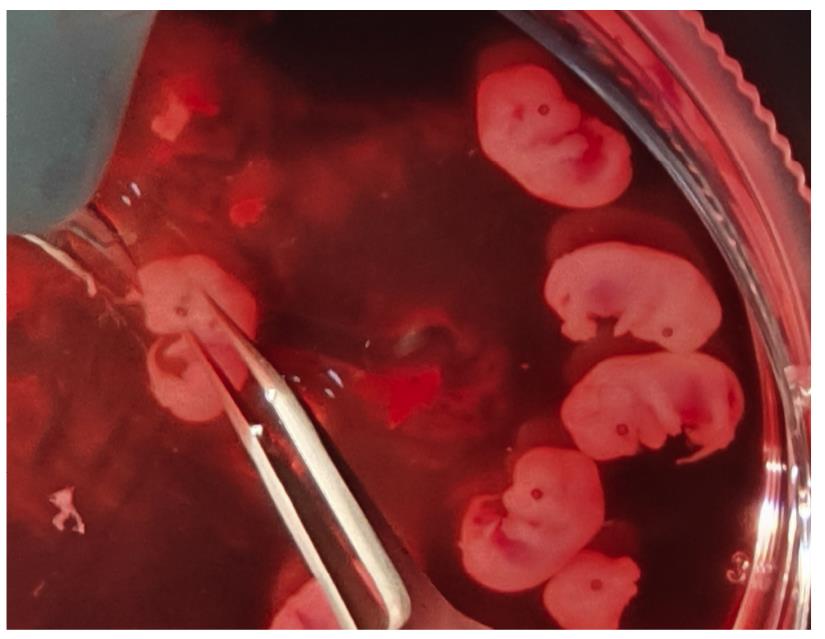
Figure 2. Dissection of mouse embryos for NEL culture. The NEL of mouse embryos was dissected using microsurgical instruments under a microscope.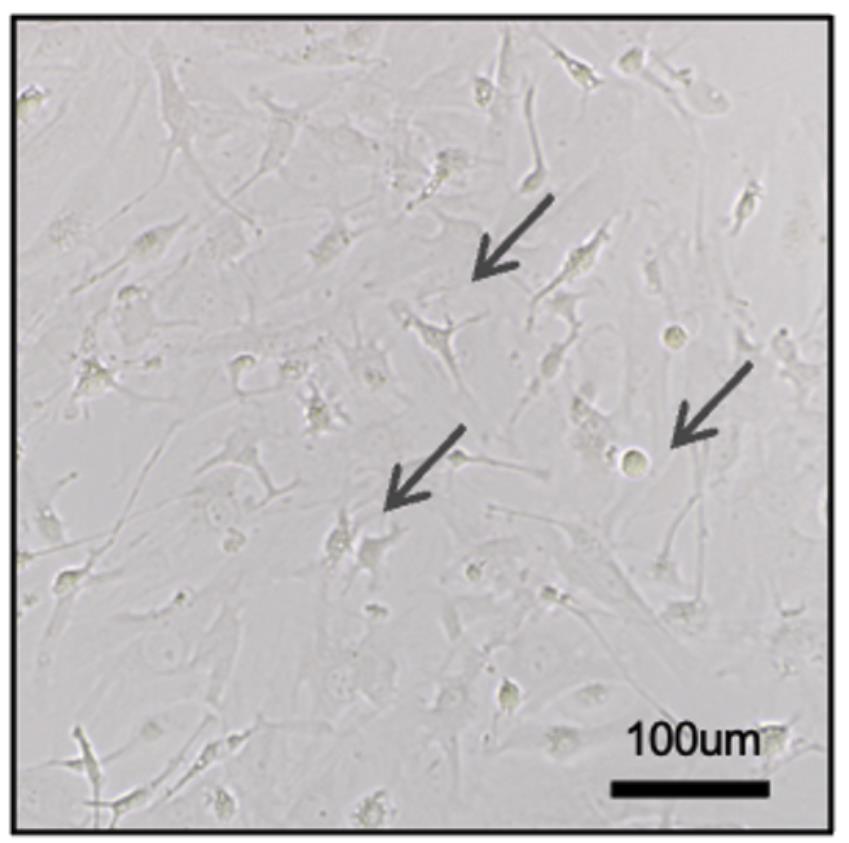
Figure 3. Expandable microglia from the NEL culture. Arrows point to the microglial progenitors that were supported by NEL. Scale bar = 100 μm.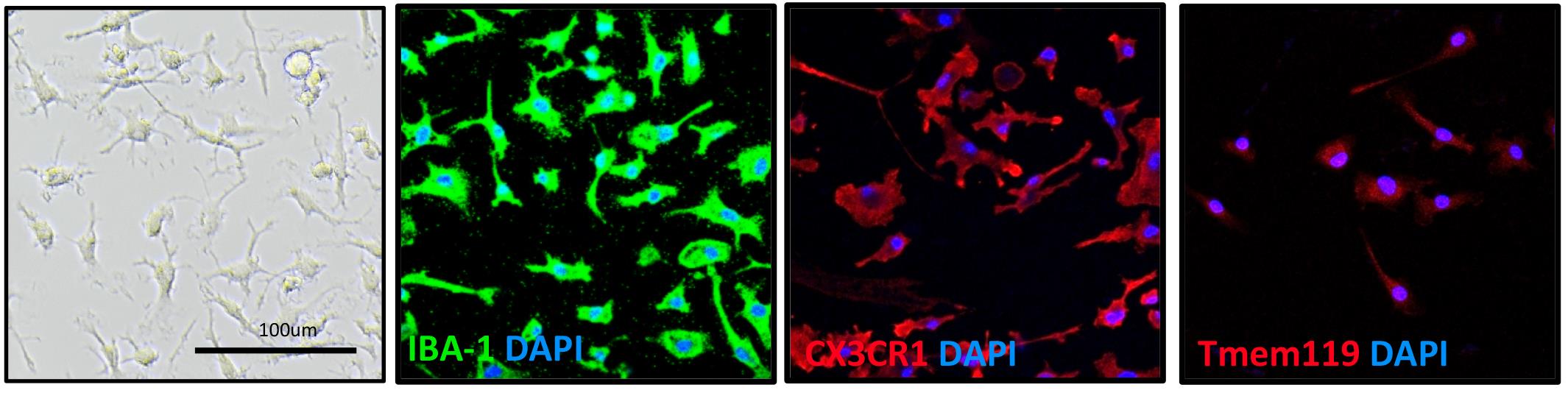
Figure 4. Isolation and purification of microglia from cultured NEL for 21 days. Cells were stained with microglial markers IBA-1, CX3CR1, and TMEM119. A bright-field microscope image shows the cell morphology. Scale bar = 100 μm.Video 1. Steps for NEL dissection and culture.
Recipes
Culture medium (500 mL)
Reagent Final concentration Amount DMEM n/a 450 mL FBS 10% 50 mL Pen/Strep
GlutaMAX
1%
0.5×
5 mL
500 µL
Total n/a 505.5 mL 70% ethanol
Reagent Final concentration Amount Ethanol (absolute) 70% 70 mL H2O n/a 30 mL Total n/a 100 mL Pentobarbital solution, 1 ampule (2 mL)
Reagent Final concentration Amount 100mg pentobarbital 3mg 2 mL Saline n/a 8 mL Total n/a 10 mL 0.25% trypsin
Reagent Final concentration Amount 2.5%Trypsin 0.25% 1 mL DPBS n/a 9 mL Total n/a 10 mL PB buffer
Reagent Final concentration Amount BSA 0.5% 0.25 g DPBS n/a 50 mL Total n/a 50 mL
Acknowledgments
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MIST) (2021M3E5D9025027). The protocol was modified and written referring to our previous paper (You et al., 2021). We appreciate Dahye Kim for providing nice illustration.
Competing interests
There is no conflict of interest.
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the CHA University (IACUC200116).
References
- You, M. J., Rim, C., Kang, Y. J. and Kwon, M. S. (2021). A new method for obtaining bankable and expandable adult-like microglia in mice. J Neuroinflammation 18(1): 294.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
You, M. J. and Kwon, M. S. (2022). A Step-by-step Protocol for Obtaining Mature Microglia from Mice. Bio-protocol 12(15): e4481. DOI: 10.21769/BioProtoc.4481.
Category
Neuroscience > Nervous system disorders
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









