- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunomagnetic Isolation and Enrichment of Microvascular Endothelial Cells from Human Adipose Tissue
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4422 Views: 3451
Reviewed by: Giusy TornilloZheng Zachory WeiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1367 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1536 Views

Non-Enzymatic Isolation of Cancer-Associated Fibroblasts From Human Prostate Tumor Explants
Giulia Gangarossa [...] Paola Chiarugi
Mar 5, 2026 74 Views
Abstract
Human adipose tissue-resident microvascular endothelial cells are not only garnering attention for their emergent role in the pathogenesis of obesity-related metabolic disorders, but are also of considerable interest for vascular tissue engineering due, in part, to the abundant, accessible, and uniquely dispensable nature of the tissue. Here, we delineate a protocol for the acquisition of microvascular endothelial cells from human fat. A cheaper, smaller, and simpler alternative to fluorescence-assisted cell sorting for the immunoselection of cells, our protocol adapts magnet-assisted cell sorting for the isolation of endothelial cells from enzymatically digested adipose tissue and the subsequent enrichment of their primary cultures. Strategies are employed to mitigate the non-specific uptake of immunomagnetic microparticles, enabling the reproducible acquisition of human adipose tissue-resident microvascular endothelial cells with purities ≥98%. They exhibit morphological, molecular, and functional hallmarks of endothelium, yet retain a unique proteomic signature when compared with endothelial cells derived from different vascular beds. Their cultures can be expanded for >10 population doublings and can be maintained at confluence for at least 28 days without being overgrown by residual stromal cells from the cell sorting procedure. The isolation of human adipose tissue-resident microvascular endothelial cells can be completed within 6 hours and their enrichment within 2 hours, following approximately 7 days in culture.
Graphical abstract:
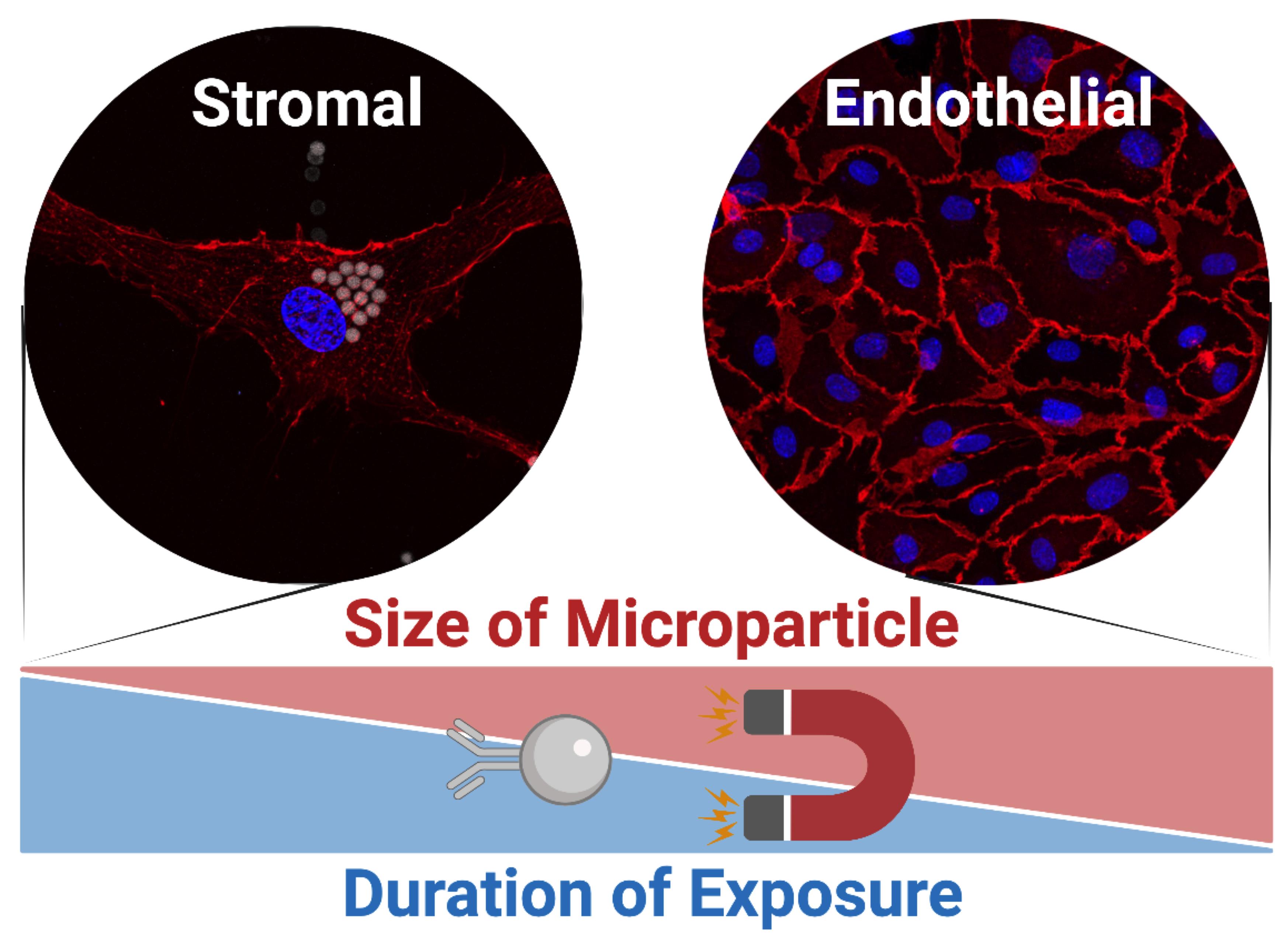
Background
White adipose tissue is not only the predominant store of energy in the body, but it is also an essential endocrine organ that helps regulate systemic metabolic homeostasis (Stern et al., 2016). While the importance of its resident microvascular endothelial cells for the growth and remodeling of the tissue is well-established, their role in the pathogenesis of obesity-related metabolic disorders is only beginning to be appreciated (Graupera et al., 2018). Our robust protocol for the acquisition of human adipose tissue-derived microvascular endothelial cells (HAMVECs) may facilitate these important investigations at the interface of vascular biology and systemic metabolic homeostasis (Antonyshyn et al., 2021). Moreover, it provides the field of vascular tissue engineering with an abundant, accessible, and uniquely dispensable source of endothelial cells, which may ultimately be used for the vascularization of engineered tissues (Rouwkema et al., 2016) and the endothelialization of small diameter vascular prostheses in an autologous, patient-specific manner (Antonyshyn et al., 2020).
Our protocol employs magnet-assisted cell sorting for the immunoselection of HAMVECs (Antonyshyn et al., 2021). Its low cost, small physical footprint, and ease-of-use make it more amenable for a number of stakeholders when compared with alternatives such as fluorescence-assisted cell sorting. Previous procedures for the acquisition of endothelial cells by magnet-assisted cell sorting have relied on differential adhesion (Hutley et al., 2001), clonal selection (Alphonse et al., 2015), and manual weeding (McGinn et al., 2004) to prevent the overgrowth of their primary cultures by residual stromal cells from the cell sorting procedure. These techniques are labour-intensive, time-consuming, and of uncertain reproducibility. We developed a procedure that does not necessitate these additional techniques to establish temporally stable cultures of HAMVECs (Antonyshyn et al., 2021).
Here, we delineate our straightforward two-staged procedure for the immunomagnetic acquisition of microvascular endothelial cells from human adipose tissue (Antonyshyn et al., 2021). The first step involves the isolation of the endothelial cells from enzymatically digested fat, and the second step involves the enrichment of their primary cultures to eliminate residual adipose tissue-derived stromal/stem cells (ASCs) from the cell sorting procedure. The stromal vascular fraction of enzymatically digested fat is depleted of CD45+ leukocytes prior to positively selecting for CD45–CD31+ HAMVECs. This sequential immunomagnetic selection is used to address challenges arising from the high prevalence of leukocytes in adipose tissue and their capacity to express characteristic endothelial markers, including CD31. The magnet-assisted cell sorting procedure employs immunomagnetic microparticles of a specific size and binding moiety, specifically comprising monodispersed superparamagnetic spheres of a 4.8 µm modal diameter conjugated with antibodies via cleavable deoxyribonucleic acid (DNA) linkers. This defined size distribution of immunomagnetic microparticles prevents their non-specific uptake by ASCs during the labeling portion of the cell sorting procedure, and the cleavable DNA linkers enable the exclusion of the superparamagnetic spheres from primary cultures where they can be more readily internalized by any residual ASCs. These are strategies that mitigate the non-specific uptake of immunomagnetic microparticles by ASCs—which is imperative for the effective isolation and enrichment of endothelial cells by magnet-assisted cell sorting. This facile two-staged procedure enables the reproducible acquisition of HAMVECs with purities ≥98%, which is sufficient to prevent their overgrowth by ASCs and facilitate their use for downstream investigations and regenerative medicine applications. Moreover, the CD45–CD31– ASCs may also be retained for downstream investigations (Antonyshyn et al., 2019), having been previously validated to meet the phenotypic criteria delineated by the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy (Bourin et al., 2013).
Materials and Reagents
Materials
Pipette tips (Corning, Axygen, catalogue numbers: T-300, T-200-Y, and T-1000-B)
Serological pipettes (Corning, Falcon, catalogue numbers: 357543, 357551, and 357525)
Petri dishes, 100 × 15 mm (Fisherbrand, catalogue number: FB0875712)
Tissue culture-treated polystyrene (Corning, Falcon, catalogue numbers: 353043 [12-well plate], 353046 [6-well plate], 353108 [T-25 flask], 353136 [T-75 flask], and 353112 [T-175 flask])
Conical tubes, 15 mL and 50 mL (Corning, Falcon, catalogue numbers: 352096 and 352070)
Round-bottom test tubes, 5 mL (Corning, Falcon, catalogue numbers: 352058)
Steritop Quick Release (0.22 µm vacuum filter; MilliporeSigma, catalogue number: S2GPT05RE)
Millex-GP Syringe Filter Unit (0.22 µm syringe filter; MilliporeSigma, catalogue number: SLGP033RS)
Syringes, 30 mL, BD Luer-Lok tip (Becton, Dickinson and Company, catalogue number: 309650)
Cell Strainers, 40 µm and 100 µm (Fisherbrand, catalogue number: 22-363-547 and 22-363-549)
Reagents
Milli-Q water (MQH2O; from Milli-Q Direct Water Purification System, see Equipment)
Dulbecco’s phosphate-buffered saline, without calcium chloride and magnesium chloride (PBS–/–; MilliporeSigma, catalogue number: D8537)
Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA; MilliporeSigma, catalogue number: E513)
Sodium hydroxide (NaOH; MilliporeSigma, catalogue number: S5881)
Bovine serum albumin solution, 35% (35% BSA stock; MilliporeSigma, catalogue number: A7979)
Ammonium chloride (NH4Cl; MilliporeSigma, catalogue number: A9434)
Potassium bicarbonate (KHCO3; MilliporeSigma, catalogue number: 60339)
Sodium bicarbonate (NaHCO3; MilliporeSigma, catalogue number: S5761)
Krebs-Ringer bicarbonate buffer (KRB powder; MilliporeSigma, catalogue number: K4002)
D-(+)-glucose (MilliporeSigma, catalogue number: G7021)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; MilliporeSigma, catalogue number: H4034)
Collagenase from Clostridium histolyticum, type II (collagenase, type II; MilliporeSigma, catalogue number: C6885)
Trypsin-EDTA solution (MilliporeSigma, catalogue no. number: T4049)
TrypLE Express (Gibco, catalogue number: 12604013)
Endothelial Cell Growth Medium-2 BulletKit (Lonza, catalogue number: CC-3162)
Biotinylated anti-CD31 antibodies (Miltenyi Biotec, catalogue number: 130-119-893)
CELLection Biotin Binder Kit (anti-biotin Dynabeads; Invitrogen, catalogue number: 11533D)
Dynabeads CD45 (anti-CD45 Dynabeads; Invitrogen, catalogue number: 11153D)
0.5 M EDTA Stock (see Recipes)
Magnet-assisted cell sorting (MACS) Buffer (see Recipes)
Erythrocyte Lysis Buffer (see Recipes)
KRB Stock (see Recipes)
Collagenase Solution (see Recipes)
Equipment
Pipettes (e.g., Eppendorf, catalogue number: 3123000900)
Pipette gun (e.g., Hirschmann Laborgeräte, catalogue number: 9907200)
Scoopula (e.g., Fisherbrand, catalogue number: 14-357Q)
Tissue Forceps, 1 × 2 teeth, 5.75” (e.g., Integra LifeSciences, catalogue number: ST6-44)
Operating Scissors, straight, sharp-blunt, 5.75” (e.g., Integra LifeSciences, catalogue number: 5-SC-16)
Analytical balance (e.g., Mettler Toledo, catalogue number: 1115010657)
pH meter (e.g., VWR, SB20 sympHony; discontinued)
Heated magnetic stir plate (e.g., IKA Works, catalogue number: 0005020001)
Analog vortex mixer (e.g., VWR, catalogue number: 10153-808)
Milli-Q Direct Water Purification System (e.g., MilliporeSigma, catalogue number: ZR0Q008WW)
Biosafety cabinet (e.g., Thermo Fisher Scientific, catalogue number: 1375)
Vacuum pump (e.g., MilliporeSigma, catalogue number: WP6111560)
CO2 incubator (e.g., Thermo Fisher Scientific, catalogue number: 3310)
Centrifuge (e.g., Eppendorf, catalogue number: 022628113)
Transmission light microscope (e.g., Leica Microsystems, catalogue number: 090-135.001)
Water bath (e.g., VWR, catalogue number: 10128-128)
Incubated shaker plate (e.g., VWR, catalogue number: 97009-894)
Magnetic stir bar (e.g., Fisherbrand, catalogue number: 14-512-127)
Beaker, 1 L (e.g., Fisherbrand, catalogue number: FB1001000)
DynaMag-5 Magnet (Invitrogen, catalogue number: 12303D)
Procedure
The immunomagnetic acquisition of HAMVECs described here is a two-staged procedure encompassing their isolation from fat and the subsequent enrichment of their cultures (Figure 1). It comprises four main steps: (A) harvesting the stromal vascular fraction from adipose tissue; (B) isolating HAMVECs from the stromal vascular fraction; (C) culturing the HAMVECs; and (D) enriching cultures of HAMVECs. The use of immunomagnetic microparticles delineated herein is key to the efficacy of the procedure (see Notes). This procedure was developed to enable the acquisition of HAMVECs from whole subcutaneous abdominal white adipose tissue but may be readily applied to lipoaspirate.
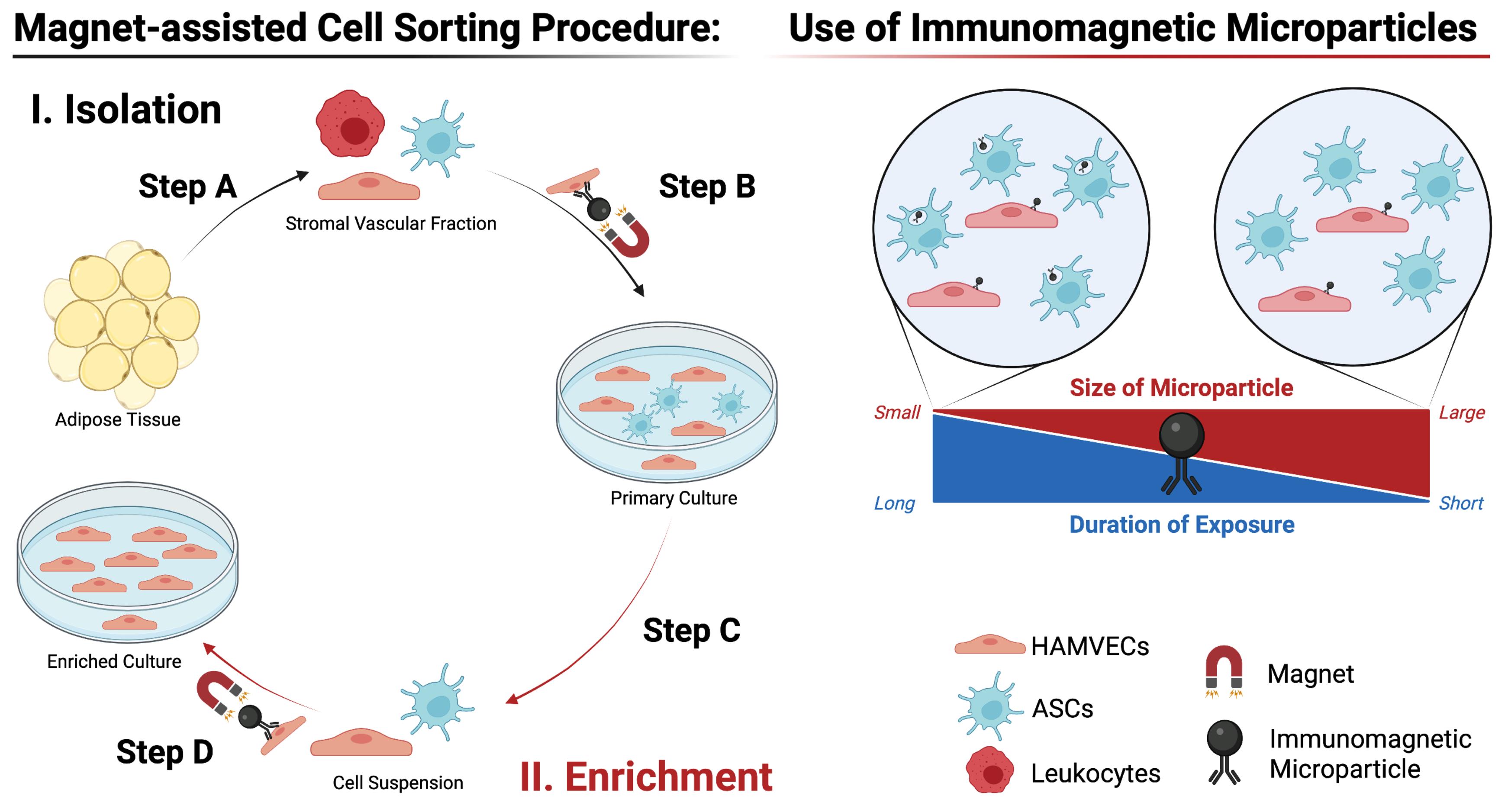
Figure 1. Immunomagnetic acquisition of endothelial cells from human adipose tissue. Schematic depicts the main steps involved in the isolation and enrichment of HAMVECs, as well as the rationale underlying the specific use of the immunomagnetic microparticles. Adapted from Antonyshyn et al. (2021).
Harvest the stromal vascular fraction from adipose tissue (Duration: 2–3 h)
Prepare the collagenase solution (see Recipes) and allow it to warm to 37°C using a water bath before processing the tissuea,b. The trypsin-EDTA solution and Endothelial Cell Growth Medium-2 may also be placed in the water bath to pre-warm to 37°C at this time. The MACS buffer (see Recipes) should be stored at 4°C in the fridge; PBS–/– and the erythrocyte lysis buffer (see Recipes) can be used as stored at room temperature (~21°C).
It is recommended that the collagenase solutions be prepared in multiples of two to help balance the centrifuge and that HAMVECs be isolated from the stromal vascular fraction derived from at least four collagenase solutions – corresponding to ~60 cm3 of fat – to mitigate the need for their protracted culture-mediated expansion (Table 1).
The volume of adipose tissue available for processing may be estimated based on its mass – measured using a balance – where the approximate density of fat is 0.9 g/cm3 (Abe et al., 2021). This estimate can be used to help determine the number of collagenase solutions that should be prepared (Table 1).
Table 1. Guide for the isolation of endothelial cells from human adipose tissue. Initial yield of HAMVECs obtained from a given amount of adipose tissue, prior to their enrichment and subsequent culture-mediated expansion, as well as the corresponding number of collagenase solutions that would need to be prepared. Isolating HAMVECs from 60–120 cm3 of fat is the most cost-efficient.
Adipose Tissue
(cm3)
Collagenase Solutions
(No.)
Plating HAMVECs
(Primary culture vessel)
Passaging HAMVECs
(Second culture vessel)
30 2 2× wells of 12-well plate 2× wells of 6-well plate 60 4 1× well of 6-well plate 1× T-25 flask 120 8 1× T-25 flask 1× T-75 flask 360 24 1× T-75 flask 1× T-175 flask
Excise adipose tissue from any skin using operating scissors and tissue forceps (Figure 2). Rinse the tissue with PBS–/– before mincing it with operating scissors in a Petri dish. Using a scoopula, add 15 cm3 of minced tissue to the pre-warmed collagenase solutiona. Incubate the solution for 60 min at 37°C under constant, gentle agitation using an incubated shaker plate.
The volume of adipose tissue being added is determined based on its displacement of the collagenase solution. Specifically, add minced tissue to the 25 mL of collagenase solution to achieve a total volume of 40 mL in each of the 50 mL conical tubes – invert the conical tubes to mix the minced tissue with the collagenase solution before assessing the volume, using the graduations demarked on the conical tubes.
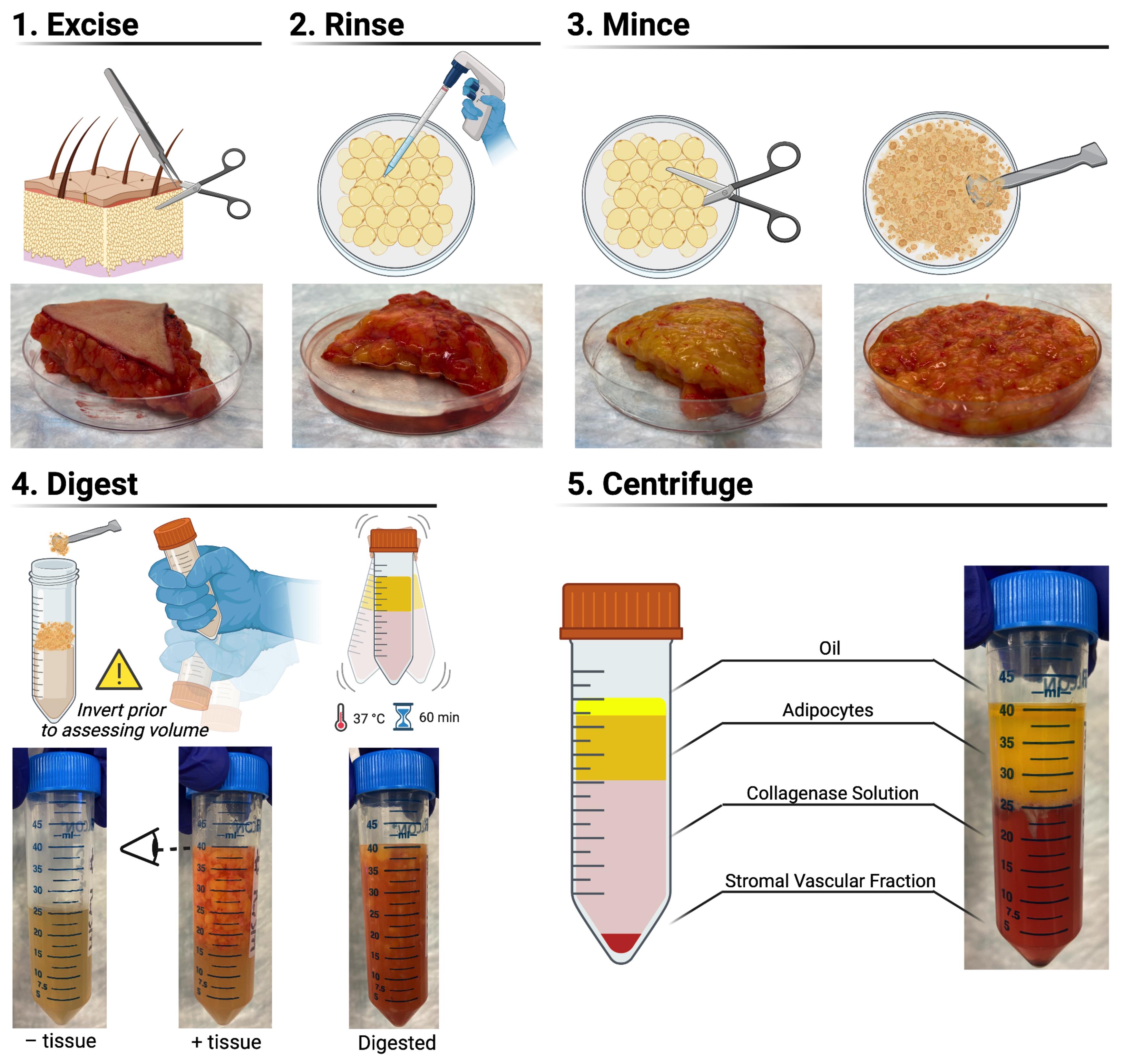
Figure 2. Harvesting the stromal vascular fraction from human adipose tissue. Schematics and representative photographs depicting the workflow involved in harvesting the stromal vascular fraction from whole subcutaneous abdominal white adipose tissue.Centrifuge the digested tissue at 1,200 × g and 21°C for 5 min. Discard the buoyant adipocytes and oil using a 25 mL serological pipette; the supernatant can be aspirated as preferred – taking care not to disrupt the loosely pelleted cells and tissue constituting the stromal vascular fraction (Figure 2). Rinse the pellet by resuspending it in 20 mL PBS–/–, centrifuging it at 1,200 × g and 21°C for 5 min, and discarding the supernatant.
Resuspend the pellet in 20 mL of pre-warmed trypsin-EDTA solution and incubate for 15 min at 37°C under constant, gentle agitation.
Add 20 mL of pre-warmed Endothelial Cell Growth Medium-2 to the solution, centrifuge it at 1,200 × g and 21°C for 5 min, and discard the supernatant. Rinse the pellet by resuspending it in 20 mL of PBS–/–, centrifuging at 1,200 × g and 21°C for 5 min, and discarding the supernatant.
Resuspend the pellet in 20 mL of erythrocyte lysis buffer and incubate for 10 min at 21°C under constant, gentle agitation.
Centrifuge the solution at 1,200 × g and 21°C for 5 min, discard the supernatant, and resuspend the pellet in 20 mL of PBS–/–. Filter the suspension through 100 µm and 40 µm cell strainers, in that order. Comprising the stromal vascular fraction, the filtrate is immediately prepared for magnet-assisted cell sorting.
Isolate CD45–CD31+ HAMVECs from the stromal vascular fraction by magnet-assisted cell sorting (Figure 3)a,b (Duration: 1.5–3 h)
The stromal vascular fraction is depleted of CD45+ leukocytes prior to positively selecting for CD45–CD31+ HAMVECs due to the high prevalence of leukocytes in adipose tissue and their capacity to express characteristic endothelial markers, including CD31.
CD45–CD31– ASCs are a by-product of this procedure and may be retained for downstream studies (see Notes).
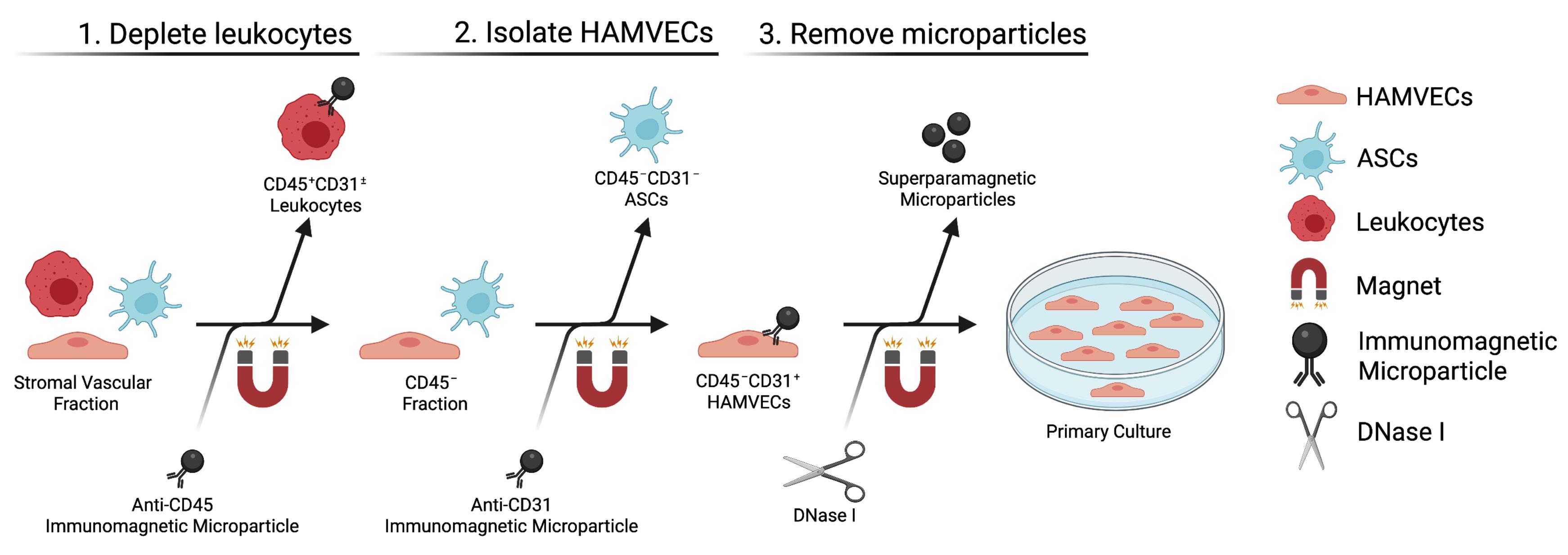
Figure 3. Isolating endothelial cells from the stromal vascular fraction by magnet-assisted cell sorting. Schematic depicting the magnet-assisted cell sorting strategy employed to isolate HAMVECs from the stromal vascular fraction of enzymatically digested adipose tissue.Pool the filtrates derived from two collagenase solutions into a single 50 mL conical tube. Centrifuge the cell suspension at 300 × g and 4°C for 5 min, discard the supernatant, and resuspend the cells in 100 µL of pre-chilled MACS buffer.
Vortex anti-CD45 Dynabeads for ≥30 s before aliquoting 50 µL into a 5 mL round-bottom test tube. Rinse the anti-CD45 Dynabeads by suspending them in 1 mL of pre-chilled MACS buffer and using the DynaMag-5 magnet to retain the Dynabeads while discarding the supernatanta.
The Dynabeads will be pulled towards the DynaMag-5 magnet quickly – wait an additional ~30 s before discarding the supernatant.
Add the cell suspension to the 5 mL round-bottom test tube containing the rinsed anti-CD45 Dynabeads, gently mix via pipetting, and incubate for 20 min at 4°C. Agitate the suspension every 5 min by gently flicking the bottom of the tube.
Add 900 µL of pre-chilled MACS buffer to the cell suspension and gently mix via pipetting before using the magnet to separate the CD45+ leukocytes from the CD45– cells. Retain the supernatant containing the CD45– cells, transferring it into a new 5 mL round-bottom test tube. Resuspend the Dynabead-bound CD45+ fraction in 1 mL pre-chilled MACS buffer, repeat the magnet-assisted cell sorting, and pool the CD45– fractionsa.
The CD45+ leukocytes can be discarded or retained for further study.
Centrifuge the CD45– cells at 300 × g and 4°C for 5 min, discard the supernatant, and resuspend the cells in 100 µL of pre-chilled MACS buffer. Add biotinylated anti-CD31 antibodies to the cell suspension to achieve a final concentration of 5 µg/mL (lot dependent), and incubate for 20 min at 4°C. Agitate the suspension every 5 min by gently flicking the bottom of the tube.
Add 3 mL pre-chilled MACS buffer to the suspension and gently mix via pipetting before centrifuging at 300 × g and 4°C for 5 min. Discard the supernatant and resuspend the cells in 100 µL of pre-chilled MACS buffer.
Vortex anti-biotin Dynabeads for ≥30 s before aliquoting 25 µL into a 5 mL round-bottom test tube. Rinse the Dynabeads by suspending them in 1 mL of pre-chilled MACS buffer and using the magnet to retain the Dynabeads while discarding the supernatant.
Add the cell suspension to the 5 mL round-bottom test tube containing the rinsed anti-biotin Dynabeads, gently mix via pipetting, and incubate for 20 min at 4°C. Agitate the suspension every 5 min by gently flicking the bottom of the tube.
Add 900 µL of pre-chilled MACS buffer to the cell suspension and gently mix via pipetting before using the magnet to separate the CD45–CD31+ HAMVECs from the CD45–CD31– ASCs. Aspirate the supernatant containing the CD45–CD31– fractiona and retain the Dynabead-bound CD45–CD31+ HAMVECs.
The CD45–CD31– fraction can be either discarded or retained to establish primary cultures of ASCs.
Resuspend the Dynabead-bound CD45–CD31+ HAMVECs in 1 mL of pre-chilled MACS buffer, repeat the magnet-assisted cell sorting, and discard the supernatant. Repeat this step once more. Resuspend the Dynabead-bound CD45–CD31+ HAMVECs in 200 µL of pre-warmed Endothelial Cell Growth Medium-2.
Reconstitute DNase I from the CELLection Biotin Binder Kit as per the manufacturer’s instructions, by adding 300 µL Releasing Buffer Component II to each vial of the Releasing Buffer Component Ia. Add 4 µL of DNase I to the Dynabead-bound CD45–CD31+ HAMVECs and incubate for 15 min at 37°C, agitating the suspension every 5 min by gently flicking the bottom of the tube.
Store reconstituted DNase I in 10 µL of aliquots at -20°C for future use.
Gently mix the cell suspension via pipetting before adding 800 µL of pre-warmed Endothelial Cell Growth Medium-2 and using the magnet to separate the Dynabeads from the CD45–CD31+ HAMVECs. Retain the supernatant to establish primary cultures of HAMVECs.
Establish, monitor, and expand primary cultures of CD45–CD31+ HAMVECsa (Duration: ~7 days)
Primary cultures of CD45–CD31– ASCs may also be established for downstream studies (see Notes).
Plate the CD45–CD31+ HAMVECs onto tissue culture-treated polystyrene at a density of ~25,000 cells/cm2 in pre-warmed Endothelial Cell Growth Medium-2, and maintain in culture at 37°C, 5% CO2 under a relative humidity of 85%a,b.
Table 1 delineates the recommended primary culture vessels onto which HAMVECs can be plated based on the amount of adipose tissue from which they were derived.
CD45–CD31– ASCs may also be plated onto tissue culture-treated polystyrene at a density of 25,000 cells/cm2, and cultured under the same conditions in the media of your choice.
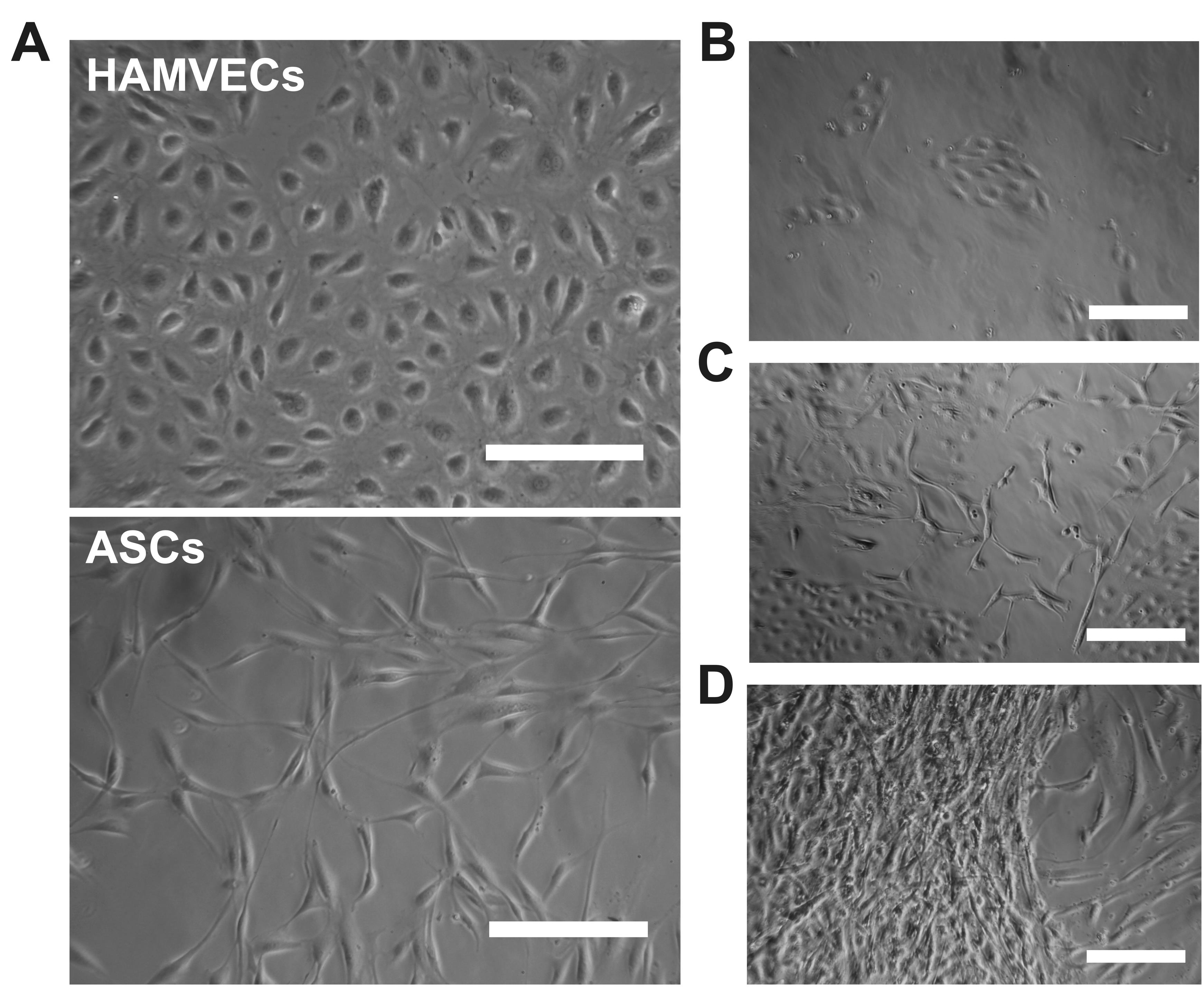
Figure 4. Monitoring primary cultures of human adipose tissue-derived microvascular endothelial cells for signs of stromal cell overgrowth. Representative photomicrographs depicting (A) the characteristic endothelial cobblestone-like morphology of HAMVECs and the spindle-shaped, fibroblast-like morphology of ASCs, as well as (B–D) different extents of contamination of primary cultures by ASCs. Scale bars represent 200 µm. Adapted from Antonyshyn et al. (2021).Exchange Endothelial Cell Growth Medium-2 every other day, monitoring primary cultures for confluence and signs of stromal cell contamination (Figure 4)a. Once the cultures are ~90% confluent, the cells are ready to be passaged into larger culture vessels – unless their overgrowth by ASCs is considerableb.
HAMVECs assume a characteristic endothelial cobblestone-like morphology after ~4 days, while residual ASCs from the magnet-assisted cell sorting procedure exhibit a spindle-shaped, fibroblast-like morphology (Figure 4A).
The presence of a small number of contaminating ASCs in between colonies of HAMVECs is typical at this stage and should not be of concern (Figure 4B, C). However, if ASCs are out-proliferating and overtaking HAMVECs (Figure 4D), proceed directly to their enrichment (Step D) rather than passaging (Steps C3–C4).
Aspirate the media, rinse cells with PBS–/–, and harvest the cells by incubating them in TrypLE Express for 10 min at 37°C a. Add an equal volume of pre-warmed Endothelial Cell Growth Medium-2 to the cell suspension, transfer it into a conical tube, and centrifuge at 300 × g and 21°C for 5 min. Discard the supernatant, resuspend the cells in pre-warmed Endothelial Cell Growth Medium-2, and re-plate them onto tissue culture-treated polystyrene in a 1:2–1:3 ratiob.
Tap the sides of the culture vessels to help release the HAMVECs. Using a serological pipette to repeatedly dispense media over the culture surface also helps recover the cells.
Table 1 provides the recommended culture vessels onto which HAMVECs can be re-plated based on their primary culture vessel.
Maintain HAMVECs in culture until they attain ~90% confluence, after which their cultures can be enriched to eliminate any residual ASCs.
Enrich primary cultures of CD45–CD31+ HAMVECs by magnet-assisted cell sorting (Duration: 1.5–2 h)
Aspirate the media, rinse cells with PBS–/–, and harvest the cells by incubating them in TrypLE Express for 10 min at 37°C. Add an equal volume of pre-warmed Endothelial Cell Growth Medium-2 to the cell suspension, transfer it into a conical tube, centrifuge at 300 × g and 21°C for 5 min, and discard the supernatant. Resuspend the cells in PBS–/–, centrifuge at 300 × g and 4°C for 5 min, and discard the supernatant.
Resuspend the cells in 100 µL of pre-chilled MACS buffer and transfer the cell suspension into a 5 mL round-bottom test tube. Add biotinylated anti-CD31 antibodies to the cell suspension to achieve a final concentration of 5 µg/mL (lot dependent), and incubate for 20 min at 4°C. Agitate the suspension every 5 min by gently flicking the bottom of the tube.
Add 3 mL of pre-chilled MACS buffer to the suspension and gently mix via pipetting before centrifuging at 300 × g and 4°C for 5 min. Discard the supernatant and resuspend the cells in 100 µL of pre-chilled MACS buffer.
Vortex anti-biotin Dynabeads for ≥30 s before aliquoting 25 µL into a 5 mL round-bottom test tube. Rinse the Dynabeads by suspending them in 1 mL pre-chilled MACS buffer and using the magnet to retain the Dynabeads while discarding the supernatant.
Add the cell suspension to the 5 mL round-bottom test tube containing the rinsed anti-biotin Dynabeads, gently mix via pipetting, and incubate for 20 min at 4°C. Agitate the suspension every 5 min by gently flicking the bottom of the tube.
Add 900 µL of pre-chilled MACS buffer to the cell suspension and gently mix via pipetting before using the magnet to separate the CD45–CD31+ HAMVECs from the CD45–CD31- ASCs. Discard the supernatant containing the contaminating ASCs.
Resuspend the Dynabead-bound CD45–CD31+ HAMVECs in 1 mL of pre-chilled MACS buffer, repeat the magnet-assisted cell sorting, and discard the supernatant. Repeat this step once more. Resuspend the Dynabead-bound CD45–CD31+ HAMVECs in 200 µL of pre-warmed Endothelial Cell Growth Medium-2.
Add 4 µL of reconstituted DNase I to the Dynabead-bound CD45–CD31+ HAMVECs and incubate for 15 min at 37°C, agitating the suspension every 5 min by gently flicking the bottom of the tube.
Gently mix the cell suspension via pipetting before adding 800 µL of pre-warmed Endothelial Cell Growth Medium-2 and using the magnet to separate the Dynabeads from the CD45–CD31+ HAMVECs. Retain the supernatant to establish cultures of HAMVECs.
Re-plate the CD45–CD31+ HAMVECs onto tissue culture-treated polystyrene in a 1:1 ratio (i.e., seed HAMVECs onto tissue culture-treated polystyrene of the same surface area from which they were derived) to establish purified cultures of HAMVECs. The HAMVECs can now be expanded or used for downstream investigationsa.
While one round of enrichment is typically sufficient in obtaining CD45–CD31+ HAMVECs with a purity ≥98%, their cultures should be monitored for signs of stromal cell contamination and overgrowth. In the event of the latter, another round of enrichment may be performed (Step D).
Data analysis
The purity of HAMVECs should be characterized by flow cytometry prior to their downstream use, as previously described (Antonyshyn et al., 2021). A purity ≥98% CD45–CD31+ is sufficient in establishing temporally stable cultures of HAMVECs, which can be expanded for >10 population doublings and be maintained at confluence for at least 28 days without being overgrown by ASCs (Figure 5). HAMVECs exhibit morphological, molecular, and functional hallmarks of endothelium, yet retain a unique proteomic signature when compared with endothelial cells derived from different vascular beds (Figure 6); that is, HAMVECs isolated using this two-staged magnet-assisted cell sorting procedure retain, at least in part, their endothelial phenotype as well as organ-specific specialization.
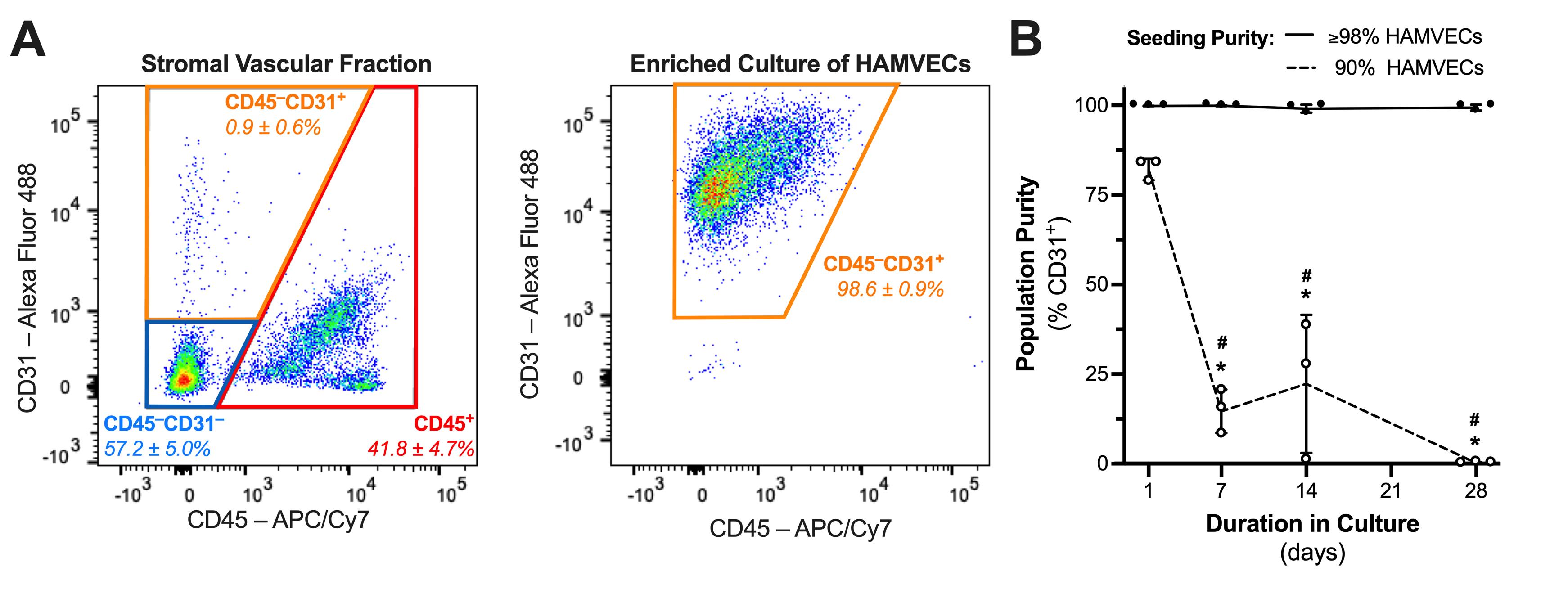
Figure 5. Cultures of human adipose tissue-derived microvascular endothelial cells established with purities ≥98% remain free of stromal cell overgrowth for at least 28 days. (A) Prevalence of CD45–CD31+ HAMVECs in the stromal vascular fraction of human subcutaneous abdominal white adipose tissue and the purity of their cultures established using the two-staged magnet-assisted cell sorting procedure. (B) Temporal stability of cultures of HAMVECs established with a purity ≥98% versus those with a purity of 90%. Adapted from Antonyshyn et al. (2021).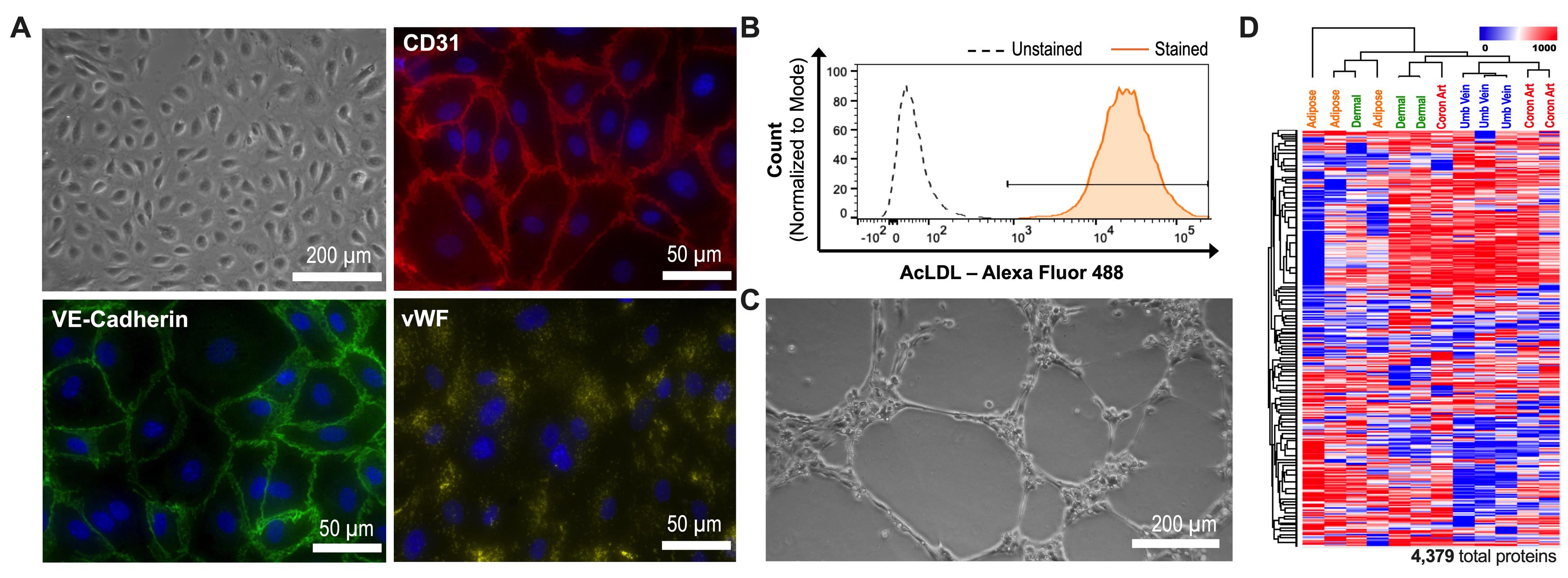
Figure 6. Human adipose tissue-derived microvascular endothelial cells exhibit morphological, molecular, and functional hallmarks of endothelium, yet retain a unique proteomic signature when compared with endothelial cells derived from different vascular beds. (A) HAMVECs exhibit a characteristic endothelial cobblestone-like morphology, and express CD31, vascular endothelial (VE)-cadherin, and von Willebrand factor (vWF). HAMVECs also perform characteristic endothelial functions, including (B) acetylated low-density lipoprotein (AcLDL) uptake and (C) angiogenic sprouting in gelled basement extract. (D) Protein expression profile of HAMVECs distinguishes them from endothelial cells derived from different vascular beds, such as the dermis, umbilical vein, and coronary artery. Adapted from Antonyshyn et al. (2021).Notes
Standard aseptic practices should be employed when conducting this procedure.
The size and binding moiety of the immunomagnetic microparticles are integral to the efficacy of this magnet-assisted cell sorting procedure, and care must therefore be taken to obtain and use the related reagents as delineated herein (see Reagents and Procedure). Immunomagnetic microparticles ≤3.9 µm in modal diameter are readily taken-up by ASCs during the labeling portion of the cell sorting procedure, while those ≥4.4 µm in diameter can be internalized by ASCs in culture (Antonyshyn et al., 2021). Accordingly, the immunomagnetic microparticles employed by this protocol have a modal diameter of 4.8 µm, which mitigates their non-specific uptake by ASCs during the labeling portion of the cell sorting procedure, and they are conjugated to antibodies through cleavable DNA linkers, which enables the exclusion of the superparamagnetic microparticles from primary cultures where they can be more readily internalized by any residual ASCs. This is imperative for the effective isolation and enrichment of HAMVECs by magnet-assisted cell sorting.
If CD45–CD31– ASCs are retained for downstream investigations, their immunophenotype, clonogenic capacity, and multipotency should be validated in accordance with the guidelines delineated by the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy (Bourin et al., 2013), as previously described (Antonyshyn et al., 2019).
Recipes
0.5 M EDTA Stock
Add EDTA to 250 mL of MQH2O, adjust pH to 8.0 using NaOH, and stir under heat (60°C) to dissolve before topping up with MQH2O to achieve a final volume of 500 mL. Sterilize via 0.22 µm vacuum filter and store at room temperature.Reagent Final concentration Amount MQH2O N/A ~406.94 mL EDTA 0.5 M 93.06 g Total N/A 500 mL
Magnet-assisted cell sorting (MACS) Buffer
Remove 9.14 mL of PBS–/– from a sterile 500 mL bottle before adding sterile 0.5 M EDTA stock and sterile 35% BSA stock. Store at 4°C.
Reagent Final concentration Amount PBS–/– N/A 491.86 mL 0.5 M EDTA Stock 2 mM 2 mL 35% BSA Stock 0.5% 7.14 mL Total N/A 500 mL Erythrocyte Lysis Buffer
Dissolve KHCO3, NH4Cl, and 0.5 M EDTA stock in 490 mL of MQH2O before topping up with MQH2O to achieve a final volume of 500 mL. Sterilize via 0.22 µm vacuum filter and store at room temperature.
Reagent Final concentration Amount MQH2O N/A ~495.28 mL 0.5 M EDTA Stock 0.1 mM 100 µL KHCO3 10 mM 0.5 g NH4Cl 0.154 M 4.12 g Total N/A 500 mL KRB Stock
Dissolve NaHCO3 and one aliquot of KRB powder in 1 L MQH2O. Sterilize via 0.22 µm vacuum filter and store at room temperature.
Reagent Final concentration Amount MQH2O N/A 1 L KRB Powder 1× 1 aliquot NaHCO3 15 mM 1.26 g Total N/A 1 L Collagenase Solution
Add collagenase, HEPES, and glucose to 50 mL conical tube and store at -20°C until needed. When ready to use, dissolve in KRB stock, add 35% BSA stock, and sterilize via 0.22 µm syringe filter. Warm to 37°C using water bath before adding minced tissue.
*Note: Final concentration of collagenase reflects that once ~15 cm3 of minced adipose tissue is added to the solution, yielding a final volume of 40 mL.
Reagent Final concentration Amount KRB Stock N/A 23.57 mL 35% BSA Stock 20 mg/mL 1.43 mL D-(+)-glucose 3 mM 13.52 mg HEPES 25 mM 148.9 mg Collagenase, type II 2 mg/mL* 40 mg Total N/A 25 mL
Acknowledgments
This work was funded by the Canadian Institutes for Health Research (grant No. 230762 & 426275). The development and validation of this protocol was previously described by Antonyshyn et al. (2021), and the procedure for harvesting the stromal vascular fraction was adapted from Flynn et al. (2006).
Competing interests
The authors have no conflicts of interest to disclose.
Ethics
Subcutaneous abdominal white adipose tissue was obtained with informed consent from patients undergoing reconstructive breast surgery at the University Health Network (Toronto, Ontario, Canada; institutional research ethics board approval No. 13-6437-CE).
References
- Abe, T., Thiebaud, R. S. and Loenneke, J. P. (2021). The mysterious values of adipose tissue density and fat content in infants: MRI-measured body composition studies. Pediatr Res 90: 963-965.
- Alphonse, R. S., Vadivel, A., Zhong, S., McConaghy, S., Ohls, R., Yoder, M. C. and Thébaud, B. (2015). The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat Protoc 10: 1697-1708.
- Antonyshyn, J. A., D’Costa, K. A. and Santerre, J. P. (2020). Advancing tissue-engineered vascular grafts via their endothelialization and mechanical conditioning. J Cardiovasc Surg 61: 555-576.
- Antonyshyn, J. A., Mazzoli, V., McFadden, M. J., Gramolini, A. O., Hofer, S. O. P., Simmons, C. A. and Santerre, J. P. (2021). Mitigating the non-specific uptake of immunomagnetic microparticles enables the extraction of endothelium from human fat. Commun Biol 4: 1205.
- Antonyshyn, J. A., McFadden, M. J., Gramolini, A. O., Hofer, S. O. P. and Santerre, J. P. (2019). Limited endothelial plasticity of mesenchymal stem cells revealed by quantitative phenotypic comparisons to representative endothelial cell controls. Stem Cells Transl Med 8: 35-45.
- Bourin, P., Bunnel, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., Redl, H., Rubin, J. P., Yoshimura K. and Gimble, J. M. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15: 641-648.
- Flynn, L., Semple, J. L. and Woodhouse, K. A. (2006). Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A 79: 359-369.
- Graupera, M. and Claret, M. (2018). Endothelial cells: new players in obesity and related metabolic disorders. Trends Endocrinol Metab 29: 781-794.
- Hutley, L. J., Herington, A. C., Shurety, W., Cheung, C., Vesey, D. A., Cameron, D. P. and Prins, J. B. (2001). Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab 281: E1037-E1044.
- McGinn, S., Poronnik, P., Gallery, E. D. M. and Pollock, C. A. (2004). A method for the isolation of glomerular and tubulointerstitial endothelial cells and a comparison of characteristics with the human umbilical vein endothelial cell model. Nephrology 9: 229-237.
- Rouwkema, J. and Khademhosseini, A. (2016). Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol 34: 733-745.
- Stern, J. H., Rutkowski, J. M. and Scherer, P. E. (2016). Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23: 770-784.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Antonyshyn, J. A., Mazzoli, V., McFadden, M. J., Gramolini, A. O., Hofer, S. O. P., Simmons, C. A. and Santerre, J. P. (2022). Immunomagnetic Isolation and Enrichment of Microvascular Endothelial Cells from Human Adipose Tissue. Bio-protocol 12(10): e4422. DOI: 10.21769/BioProtoc.4422.
Category
Biological Engineering > Biomedical engineering
Medicine > Cardiovascular system
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








