- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Standardized Peripheral Nerve Transection Method and a Novel Digital Pressure Sensor Device Construction for Peripheral Nerve Crush Injury
Published: Vol 12, Iss 5, Mar 5, 2022 DOI: 10.21769/BioProtoc.4350 Views: 3026
Reviewed by: Alessandro DidonnaHélène LégerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Procedure for Reliable and Long-Lasting Ex Vivo Recordings of Sciatic Nerve Activity in Mice
Shani Berkowitz [...] Jérôme Joël Devaux
Mar 5, 2025 2547 Views

Electrophysiological Evaluation of a Sciatic Nerve Degree III Injury Model in Rats
Linyu Chen [...] Na Dou
May 20, 2025 1800 Views

Whole-Mount Immunostaining for the Visual Separation of A- and C-Fibers in the Study of the Sciatic Nerve
Valeriia Ustymenko [...] Nana Voitenko
Dec 5, 2025 1422 Views
Abstract
Peripheral nerve injury (PNI) is common in all walks of life, and the most common PNIs are nerve crush and nerve transection. While optimal functional recovery after crush injury occurs over weeks, functional recovery after nerve transection with microsurgical repair and grafting is poor, and associated with permanent disability. The gold-standard treatment for nerve transection injury is microsurgical tensionless end-to-end suture repair. Since it is unethical to do experimental PNI studies in humans, it is therefore indispensable to have a simple, reliable, and reproducible pre-clinical animal model for successful evaluation of the efficacy of a novel treatment strategy. The objective of this article is two-fold: (A) To present a novel standardized peripheral nerve transection method in mice, using fibrin glue for modeling peripheral nerve transection injury, with reproducible gap distance between the severed nerve ends, and (B) to document the step-wise description of constructing a pressure sensor device for crush injury pressure measurements. We have successfully established a novel nerve transection model in mice using fibrin glue, and demonstrated that this transection method decreases surgical difficulties and variability by avoiding microsurgical manipulations on the nerve, ensuring the reproducibility and reliability of this animal model. Although it is quite impossible to exactly mimic the pathophysiological changes seen in nerve transection with sutures, we hope that the close resemblance of our novel pre-clinical model with gold-standard suturing can be easily reproduced by any lab, and that the data generated by this method significantly contributes to better understanding of nerve pathophysiology, molecular mechanisms of nerve regeneration, and the development of novel strategies for optimal functional recovery. In case of peripheral nerve crush injury, current methods rely on inter-device and operator precision to limit the variation with applied pressure. While the inability to accurately quantify the crush pressure may result in reduced reproducibility between animals and studies, there is no documentation of a pressure monitoring device that can be readily used for real-time pressure measurements. To address this deficit, we constructed a novel portable device comprised of an Arduino UNO microcontroller board and force sensitive resistor (FSR) capable of reporting the real-time pressure applied to a nerve. This novel digital pressure sensor device is cheap, easy to construct and assemble, and we believe that this device will be useful for any lab performing nerve crush injury in rodents.
Keywords: Peripheral nerve injuryBackground
Peripheral nerve injury (PNI) represents a major health problem that often leads to significant functional impairment and permanent disability, resulting in annual costs of billions of dollars (Noble et al., 1998; Robinson, 2000; Foster et al., 2019; Karsy et al., 2019). PNI can range from simple compression to injuries with an intervening gap, where the nerve is completely separated (Campbell, 2008; Robinson, 2004; Caillaud et al., 2019). While optimal functional recovery after crush injury (axonotmesis, loss of axon continuity with preservation of nerve sheath) occurs over a period of 4–6 weeks (Hare et al., 1992; Moore et al., 2012; Caillaud et al., 2019), functional recovery after a severed nerve injury (neurotmesis, where the nerve itself is transected) with microsurgical repair and grafting is poor (Zochodne, 2012; Faroni et al., 2015), and full functional recovery is rare.
The available treatment for a completely severed nerve is end-to-end repair, nerve grafting, nerve conduits, or nerve transfer (Siemionow and Brzezicki, 2009; Isaacs, 2013). Poor functional recovery after these highly advanced microsurgical and reconstructive techniques could be partly due to the failure of these techniques to address the complex cellular and molecular events associated with PNI and repair (Zochodne, 2012; Faroni et al., 2015). To improve functional results for patients with PNIs, it is important to investigate multiple critical factors that are intimately related to the success of nerve regeneration and target innervation. While experimental studies to uncover mechanistic insights of PNI are impossible and unethical in humans, we recently established a novel standardized peripheral nerve transection technique in mice, which we refer to as Stepwise Transection and fibrin Glue (STG) (Lee et al., 2020), to investigate the mechanisms underlying poor functional outcomes. In the STG method, the nerve is first incompletely cut to 80% of its width, to prevent gap formation. Then, fibrin glue is applied around the laceration site, and the remaining 20% of the nerve is completely transected before the complete clotting of the glue. The STG method demonstrated post-injury pathophysiological changes comparable with the gold-standard suture repair, in terms of nerve histology and functional recovery (Lee et al., 2020). The STG method decreased surgical difficulties and variability by avoiding microsurgical manipulations on the nerve, thus ensuring the reproducibility and reliability of this animal model. Our novel transection method has several advantages as an experimental model when compared with current gold standard end-to-end neurorrhaphy, including: (i) standardized gap distance without microsurgical suturing, (ii) simplicity and reproducibility with faster procedure time, and (iii) lack of confounding factors associated with multiple suturing sites.
The sciatic nerve crush injury in rodents is a century-old model, widely used to study peripheral nerve regeneration and the potential beneficial effects of novel therapeutic strategies. Although crush injury can recover well within weeks in animal models (Hare et al., 1992; Moore et al., 2012), severe crush injury in humans with neuroma formation requires surgical intervention (Tos et al., 2012; Caillaud et al., 2019). Different tools (jeweler’s forceps, forceps with custom jig, malleus nipper, or needle driver), forces (~5 MPa to ~190 MPa), and duration (3 sec to 10 min) are used to create crush injuries (Chen et al., 1992; Bridge et al., 1994; Alant et al., 2012; Savastano et al., 2014; Tseng et al., 2016; Geary et al., 2017; Yue et al., 2019; Wandling et al., 2021), and there are concerns regarding the severity of crush injury pressure and the reproducibility of the method used. There have been limited attempts to quantify the pressure applied during crush injury in rodents, using a thin cell load for digital pressure display (Alant et al., 2012; Chen et al., 1992), and pressure-sensitive films for later analysis (Geary et al., 2017). However, there is no documentation of a pressure monitoring device that can be readily used for real-time pressure measurements. Importantly, operator precision and inter-device reliability may limit the standardization of the pressure applied and reproducibly of the findings. To address this gap, we constructed a novel portable device comprised of an Arduino Uno microcontroller board and force sensitive resistor (Figure 1), capable of reporting the real-time pressure applied to a nerve during crush injury (Wandling et al., 2021). We believe that the ability to deliver a more precise and quantifiable crush injury pressure using our novel device will increase standardization of crush injury and its reliability, leading to increased reproducibility of findings in the literature.
The objective of this article is two-fold: (A) To present a novel standardized peripheral nerve transection method in mice, that uses fibrin glue for modeling peripheral nerve transection injury with a reproducible gap distance between the severed nerve ends (Figures 2–3), and (B) to document the step-wise description of constructing a pressure sensor device (Figures 4–17) for crush injury pressure measurements.
Materials and Reagents
For the Stepwise Transection with fibrin Glue
Protective gear (sterile surgical gloves, goggles, lab coat, mask, hair cap)
1 mL Sub-Q syringe (Beckton, Dickinson Company, catalog number: 309597) and 1 mL U-100 Insulin syringe (Beckton, Dickinson Company, catalog number: 329420)
3MTM Micropore Surgical Paper tape (Fisher Scientific,catalog number: 19-061655)
Sterile Cotton Tipped Applicator (Puritan Medical Products, catalog number: 25-806 2WC) and CurityTM Gauze Pads (Covidien LLC, catalog number: 3381)
Autoclaved draping sheets and autoclaved surgical instruments (listed in Equipment section, as shown in Figure 2)
Surgical staples (3M Precise Disposable Skin Stapler, DS-25; 3M Health Care, MN, USA) for wound closure
Clean animal cage with food and water bottle
Animal cage warming pad (Right Temp Jr., catalog number: RT-JR-15 Kent Scientific, CT, USA) or warming station (Premiere Slide Warmer, Model XH-2004, available at Amazon)
10-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) weighing 20–25 g
Ketamine (60 mg/kg) and Xylazine (4 mg/kg) mixture (supplied by animal care facility)
Lubricant Ophthalmic ointment (Sterile Artificial Tears, Akorn Animal Health, Lake Forest, IL, USA)
Alcohol Prep (saturated with 70% ethanol; WebcolTM Alcohol Prep, catalog number: 5110, Covidien LLC, MA, USA) and McKesson 10% povidone-iodine solution (McKesson Corporation, catalog number: 854301PA)
Fibrin glue, consisting of fibrinogen and thrombin (Fibrin Sealant TISSEEL; Baxter Healthcare Corporation, catalog number: 1504516)
Slow release buprenorphine injection (0.05 mg/kg) (supplied by animal care facility)
Arduino Uno R3 (Arduino)
Micro SD TF card adapter reader module 6 pin (HiLetgo)
Standard LCD 16x2 Character Display Module (ELEDIY)
Micro SD card 32 GB (Samsung)
Prototype PCB shield board for Arduino Uno R3 (CZH-LABS)
Force sensitive resistor (FSR) (Flexiforce, A201, 4N)
Single row male PCB pin connectors (Honbay)
Toggle on/off switch (Hillman; 427680)
10 KOhm potentiometer (ELEDIY)
10 KOhm resistor (Elegoo)
220 Ohm resistor (Elegoo)
9V battery clip with 2.1 mm × 5.5 mm male DC plug (Corpco)
9V battery (Elegoo)
20-gauge solid wire (Lowes)
22-gauge stranded wire (Lowes)
Electrical tape (Lowes)

Figure 1. Materials for pressure sensor construction.
Equipment
For the Stepwise Transection with fibrin Glue
Operating microscope (Precision Stereo Zoom Binocular Microscope, Model PZMIII, World Precision Instruments, FL, USA)
Small animal scale (OHAUS, Model CS200)
Mouse clippers (All-in-one trimmer, Philips Norelco, Multigroom 3000)
Fine scissors (Figure 2A; Miltex, catalog number: MH18-1476, Integra LifeSciences, NJ, USA)
Surgical scissors (Figure 2B; Tekno-Medical, catalog number: tK8272-4)
Dissecting forceps (Figure 2C; Integra LifeSciences, catalog number: MH17-301)
Jeweler’s forceps (Figure 2D; Integra LifeSciences, catalog number: MH17-305)
Microscopic scissors (Figure 2E; Miltex, catalog number: MH18-1620, Integra LifeSciences, NJ, USA)
(Optional) Small Alm self-retaining retractor (Figure 2F; Tekno-Medical, catalog number: tK17330-07)
Heating pad (Right Temp Jr., catalog number: RT-JR-15, Kent Scientific, CT, USA)
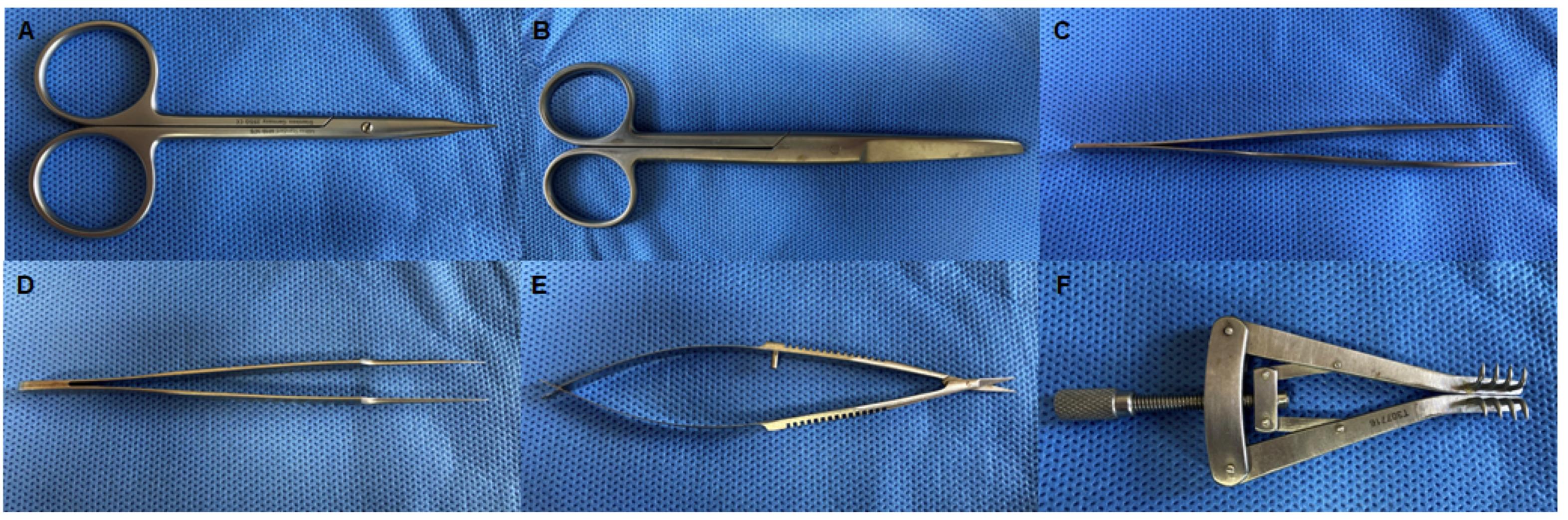
Figure 2. Surgical instruments for nerve transection.
Wire cutters (Lowes)
Needle nose pliers (Lowes)
Wire strippers (Lowes)
Soldering iron (Vastar)
Soldering wire (Tin/Lead; 60/40) (Flux 2.0%) (Vastar)
Flux soldering paste (Rubyfluid)
Software
For the Pressure Sensor
PrecisionPinch4.0 (Grant D Wandling, https://github.com/gwandling/PrecisionPinch)
Procedure
For the Stepwise Transection with fibrin Glue (Needs one surgeon and one assistant):
Sanitize the surgical area with 70% ethanol, and organize the packets of autoclaved surgical instruments and surgical drapes in a tray.
Anesthetize the mouse with an intraperitoneal injection of ketamine/xylazine mixture (0.1 mL/10 g) in the animal preparation area.
Assess the depth of anesthesia by at least one indicator of deep pain recognition (pedal reflex, pinna reflex, etc.) to ensure adequate anesthesia.
Apply ophthalmic ointment to both eyes to prevent desiccation.
Remove hair in the right or left hind limb, and sterilize the surgical site thrice, by using alcohol prep (saturated with 70% ethanol) and povidone-iodine solution (10%) alternately.
Place the mouse under an operating microscope with the hindlimb in semi-flexion posture, using surgical skin tape (Figure 3A).
Put on the sterile surgical gloves in an aseptic manner.
Cover the mouse with sterile drapes and expose the surgical site (Figure 3B). Unpack the autoclaved surgical instruments on another sterile drape.
Make a skin incision along the gluteal fold (about 3 cm), and split the gluteal muscle using scissors and forceps, to expose the sciatic nerve (Figure 3C–D).
Do not free the sciatic nerve from the surrounding connective tissue, as this contributes to prevent gap formation after nerve transection.
Under an operating microscope, using the fine microscopic scissors cut the nerve incompletely to 80% of its width, to prevent gap formation (Figure 3E).
Apply 7–10 µL of fibrinogen firstly, and 7–10 µL of thrombin at the transection site.
As soon as possible, cut the remaining nerve completely before complete clotting of the fibrin glue applied (Figure 3F).
Close the wound with surgical staples and inject buprenorphine (0.05 mg/kg) subcutaneously.
Return the mouse to a clean cage and keep the cage on a heating pad until the mouse wakes up.
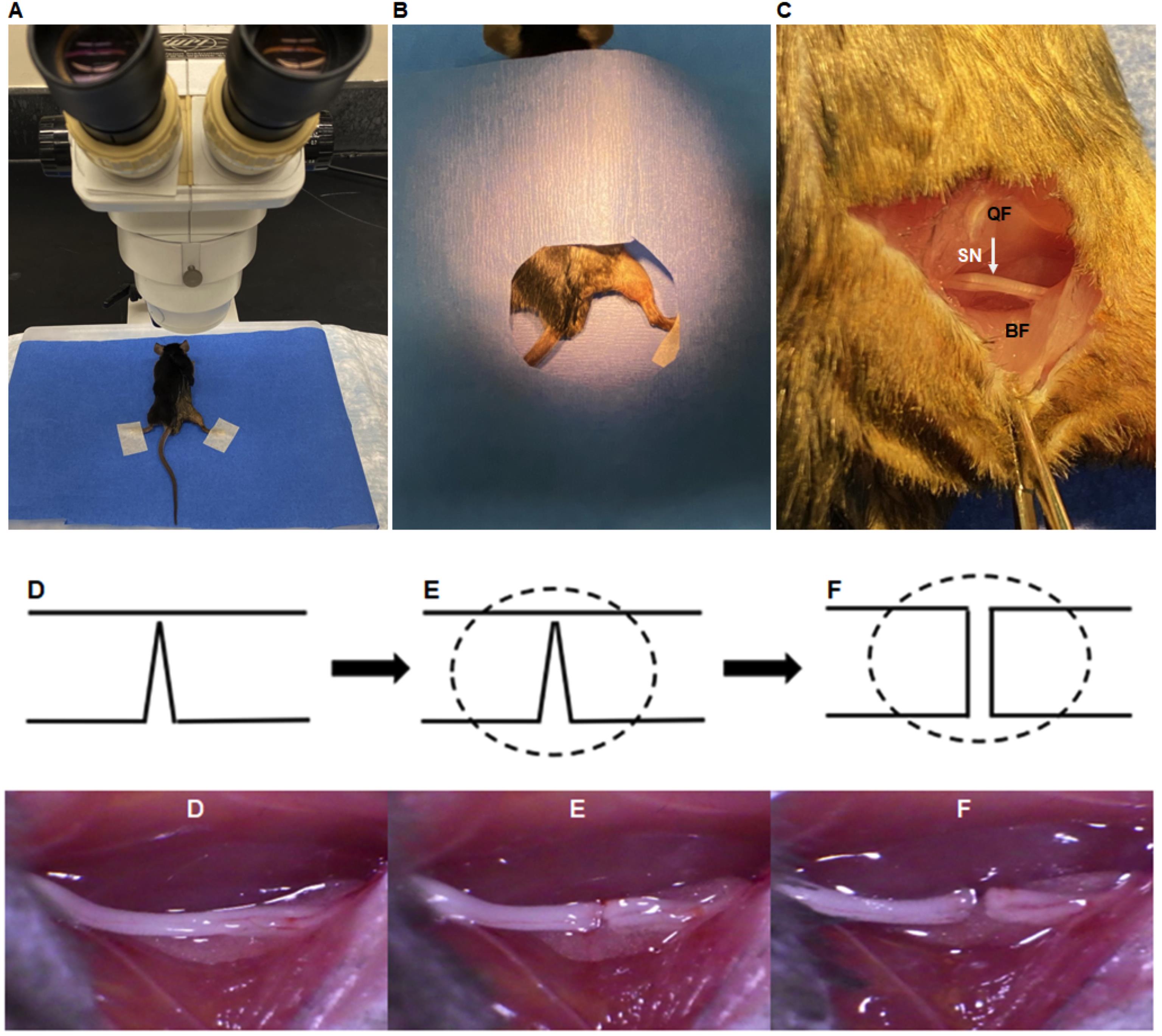
Figure 3. Stepwise nerve transection with fibrin glue. A. Position of the prepped mouse under the operating microscope. B. Position of the right hindlimb surgical site within the window of autoclaved drape sheet. C. Exposure of the sciatic nerve (SN) between the biceps femoris (BF) and the quadriceps femoris (QF) muscles. D. Exposed sciatic nerve and its position before transection. E. Cutting ~80% of the sciatic nerve. E. Cutting the remaining ~20% of the nerve completely.
Device assembly
All connections are made by soldering. See pinout diagram for connection overview (Figure 4).
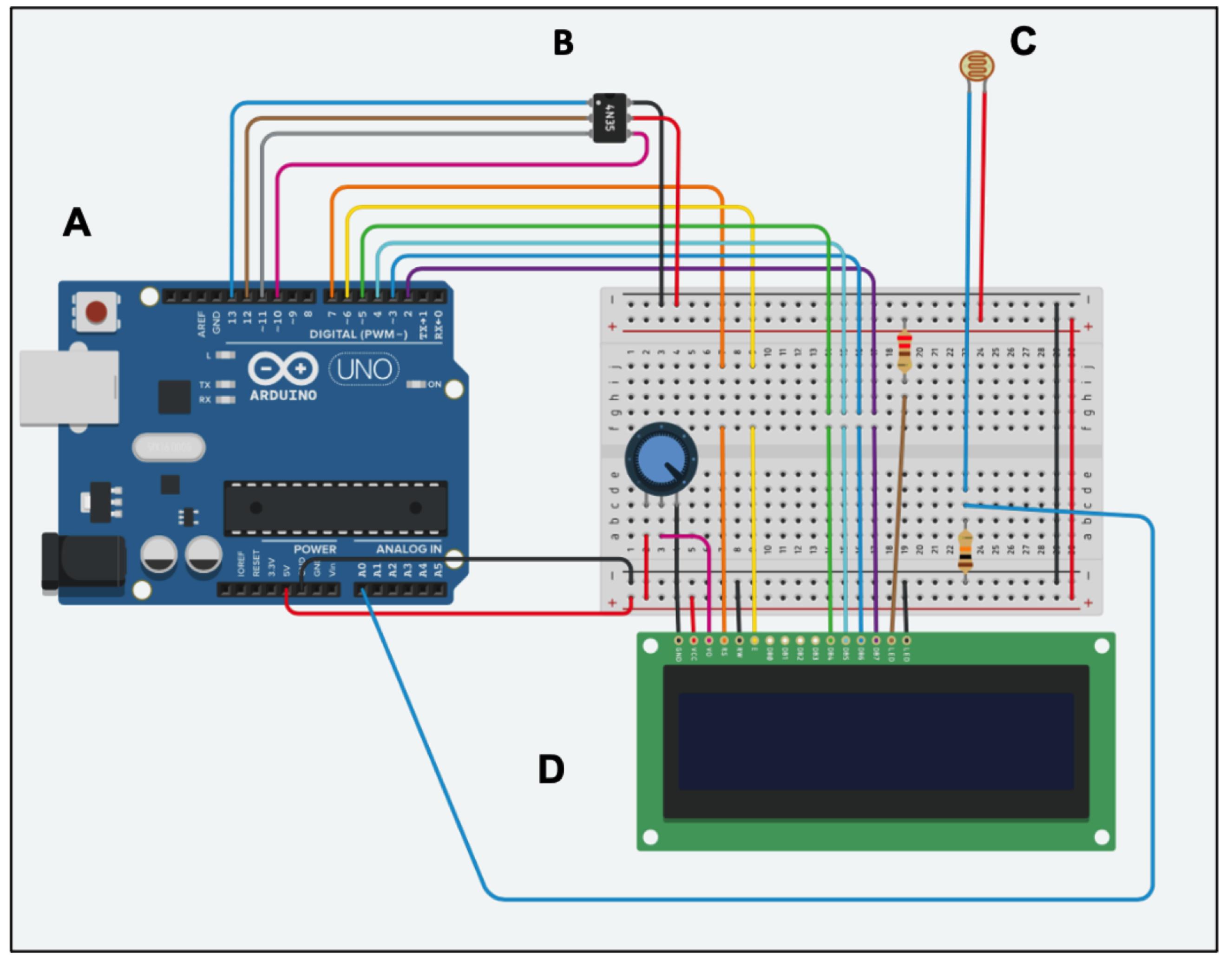
Figure 4. Pinout diagram. Schematic representation of PrecisionPinch system containing Arduino UNO (A), Micro SD card adapter (B), force sensitive resister (FSR) FlexiForce A301 (C), and 16x2 LCD screen (D).Connect male pin connectors to PCB shield board (Figure 5).
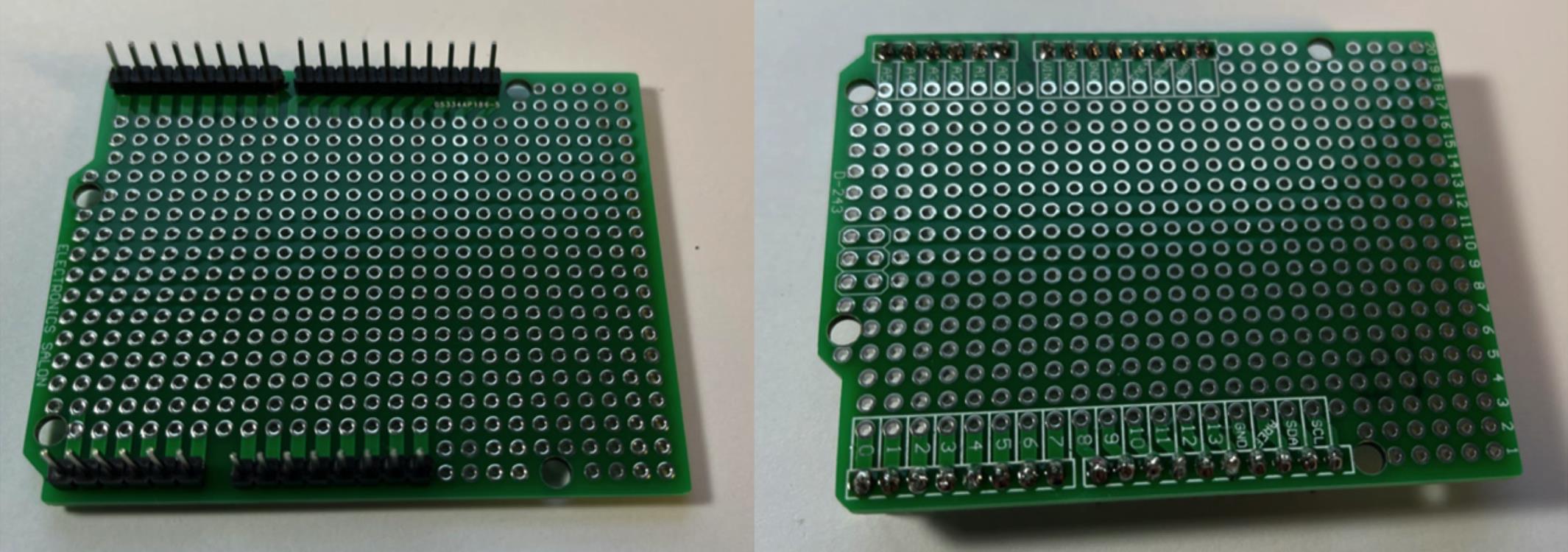
Figure 5. PCB shield board and pin connectors.Use pliers to straighten out the pins on the Micro SD TF card adapter reader module, and connect them to board (Figure 6).
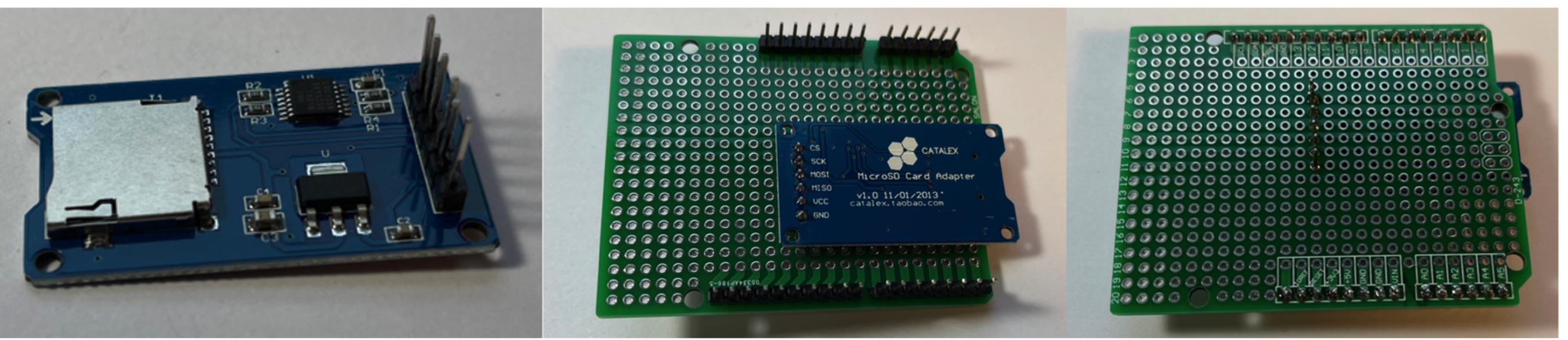
Figure 6. Connect Micro SD card adapter.Use 20-gauge solid wire to connect the Micro SD card adapter reader module to the appropriate locations: GND to GND, MISO to D12, MOSI to D11, SCK to D13, CS to D10. Solid wire is easier to solder. Connect a longer wire (~15 cm in length) to VCC. This will later be joined to +5V (Figure 7).
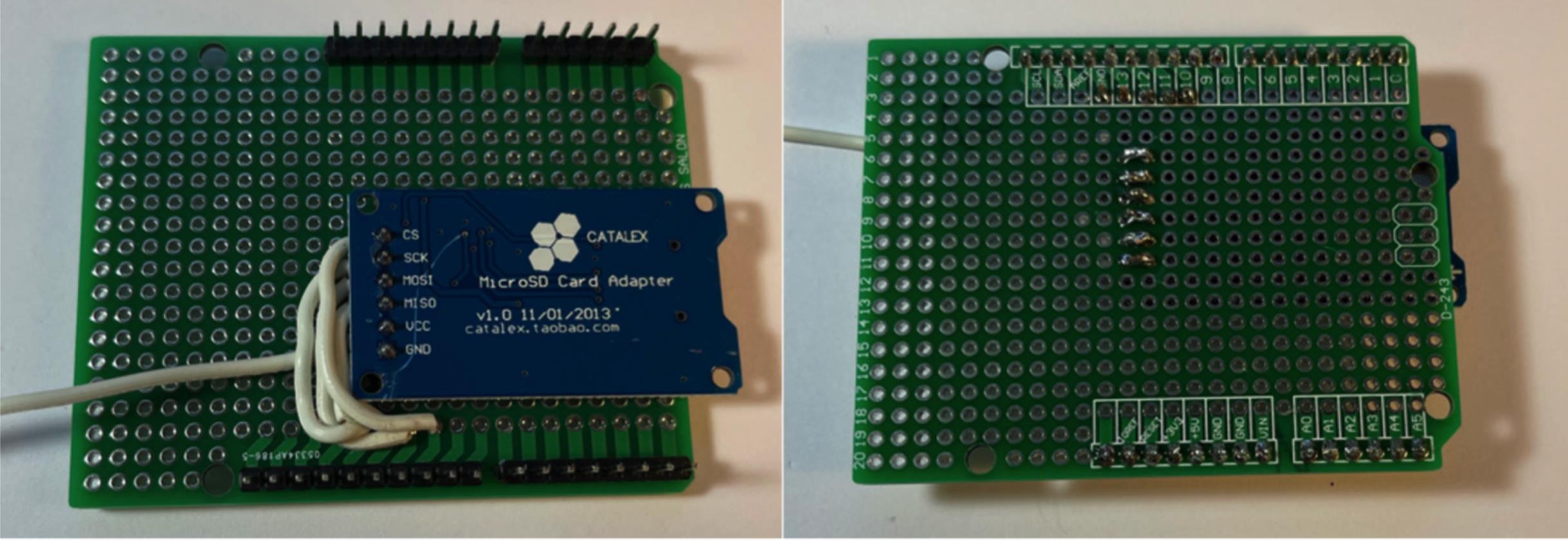
Figure 7. Connect Micro SD card adapter to appropriate locations on board.Connect six lengths of 20-guage wire (~15 cm in length) to the digital output connections of the board at D2–D7. Leave the other end to be attached later (Figure 8).
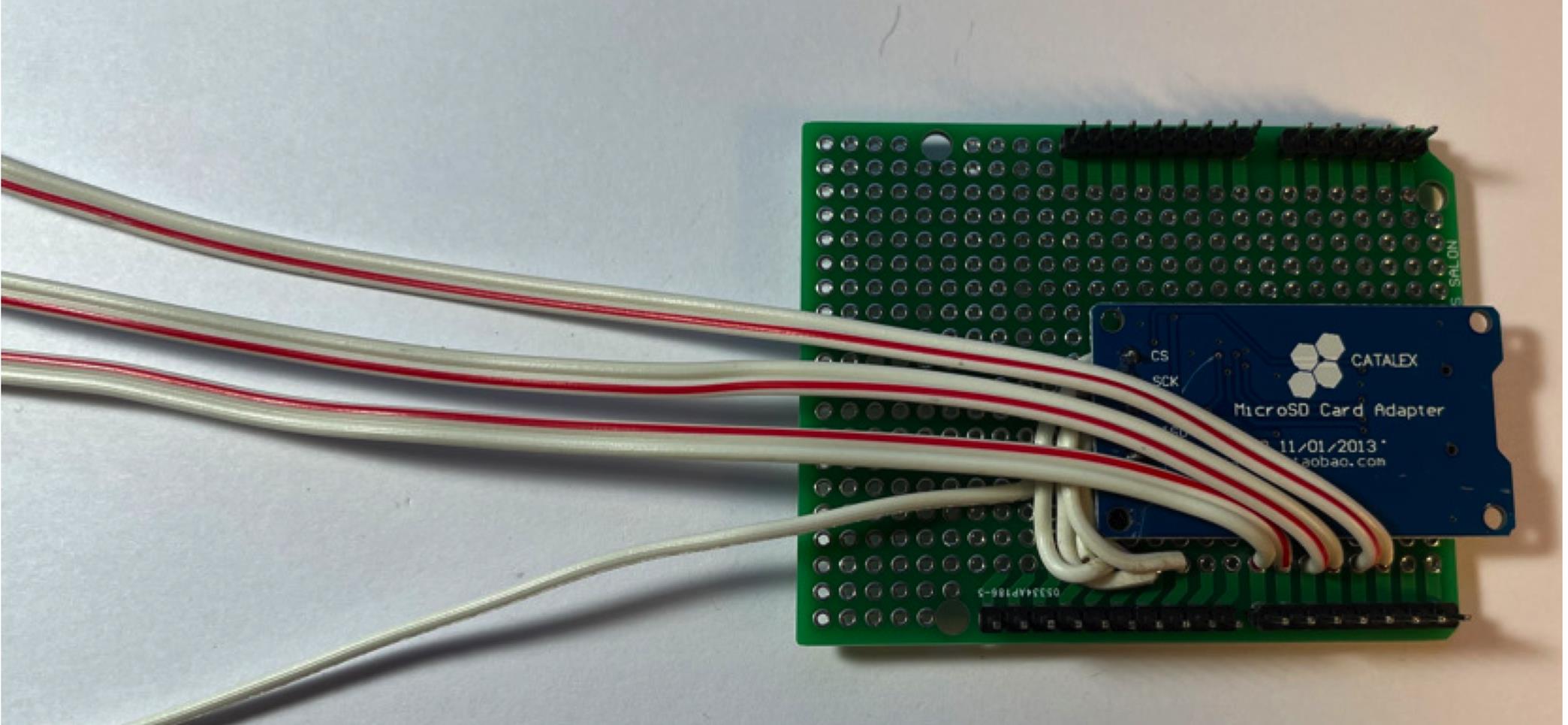
Figure 8. Connect wire to digital outputs.Connect male pin connectors to LCD display, then solder connectors to board (Figure 9).
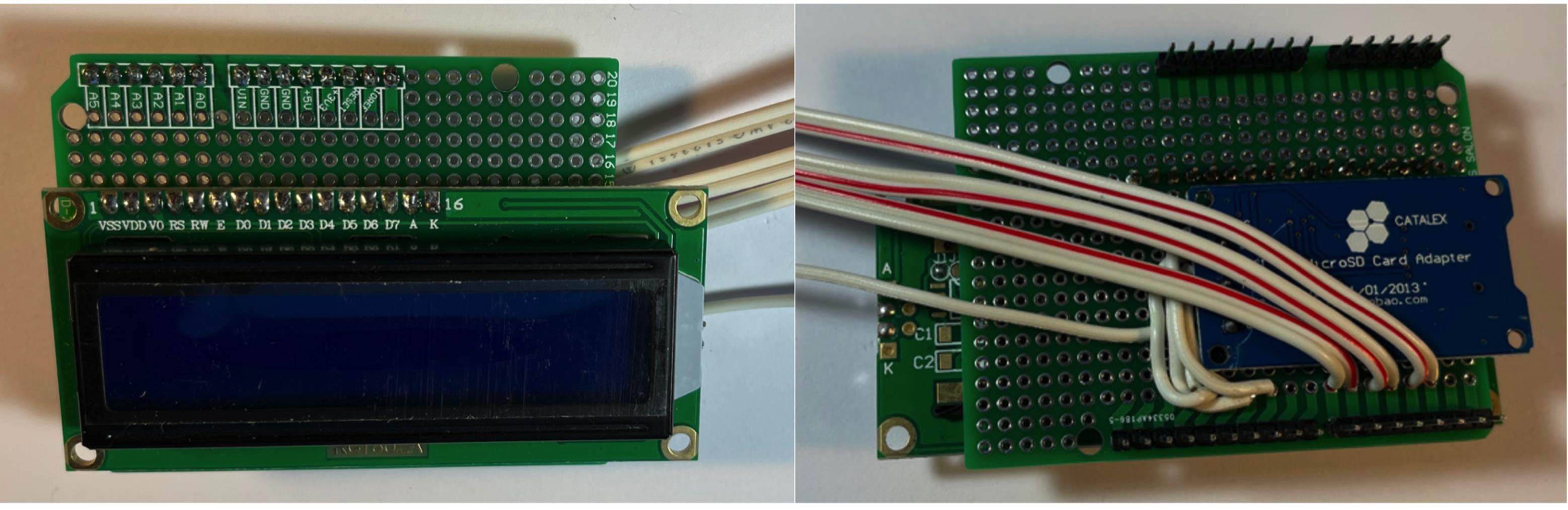
Figure 9. Solder in LCD display.Connect LCD VCC to +5V, then add on 10 KOhm potentiometer by connecting left pin to +5V, middle pin to LCD V0, and right pin to VSS (Figure 10). Refer to pinout diagram for clarification.
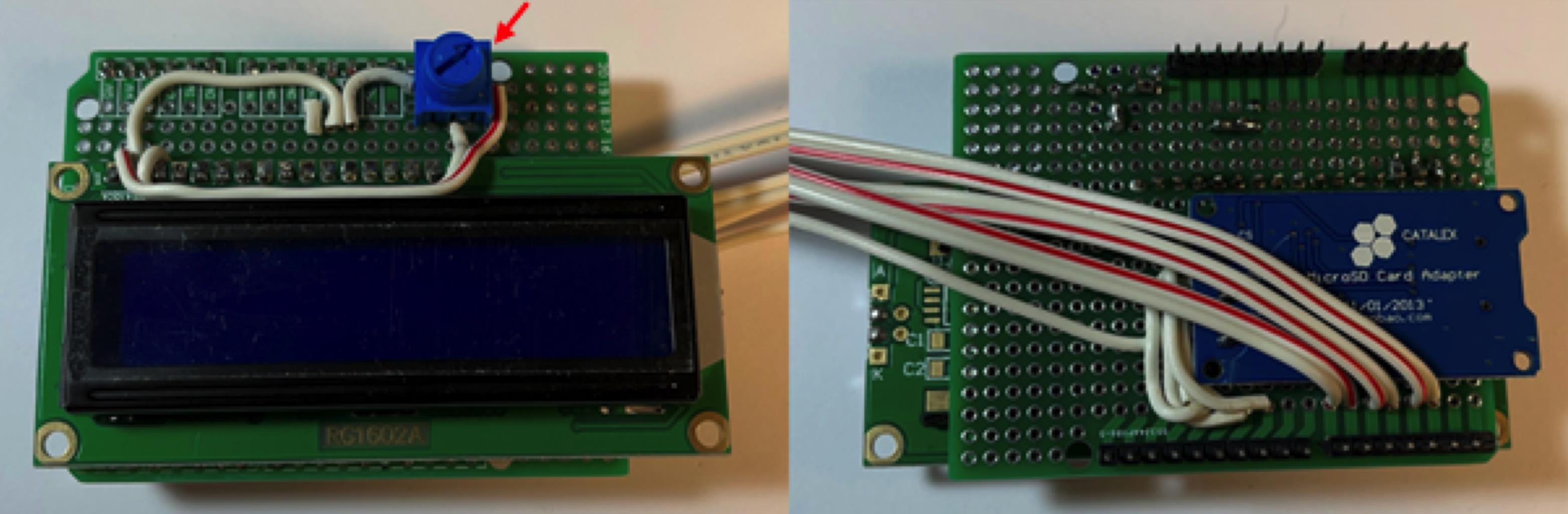
Figure 10. Connect potentiometer (red arrow) and power to the LCD display.Connect the 220 Ohm resistor (red arrow) to LCD D15 and +5V (Figure 11). Refer to pinout diagram for clarification.
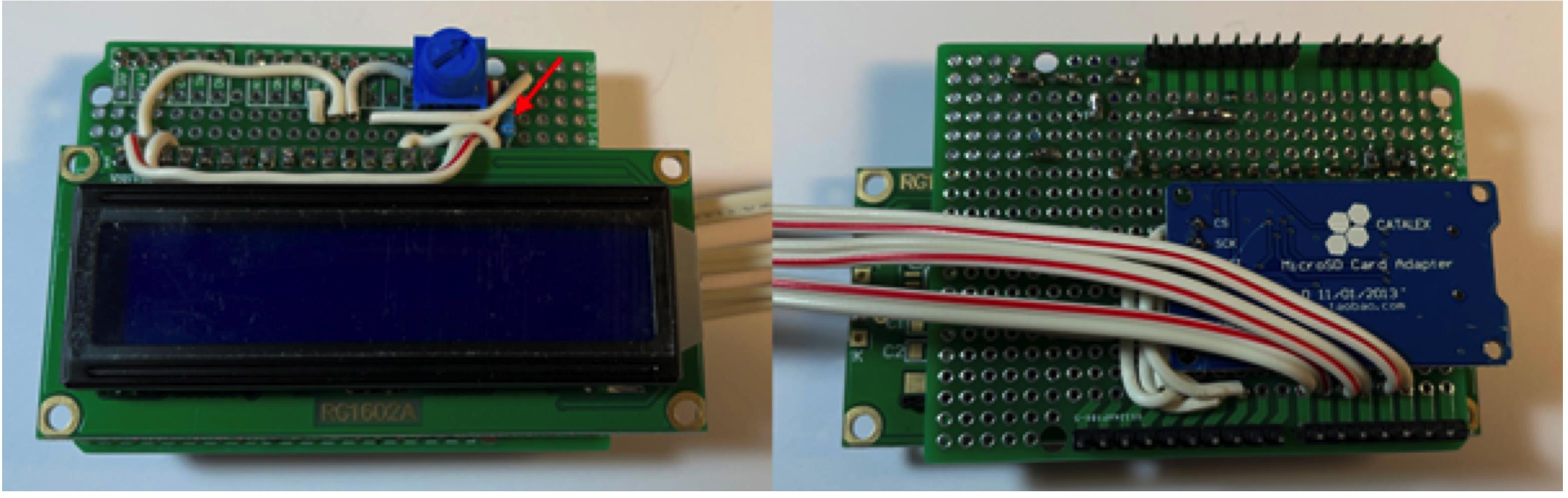
Figure 11. Connect the 220 Ohm resistor.Connect the 10 KOhm resistor (red arrow) to the board (Figure 12).
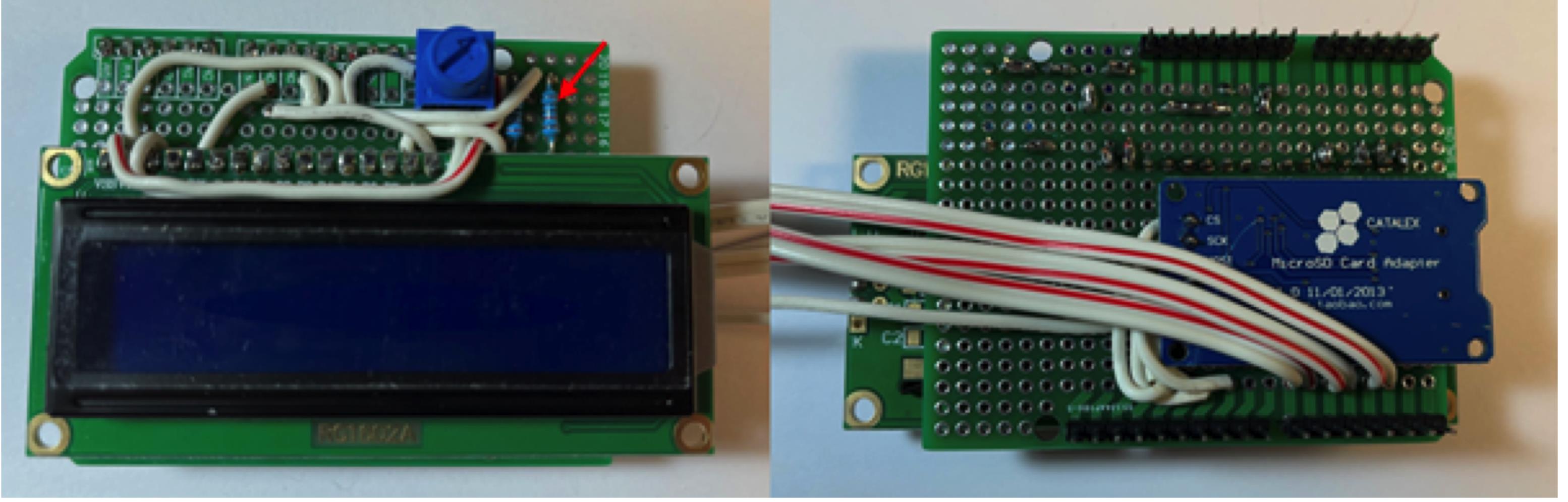
Figure 12. Connect 10 KOhm resistor.Connect stranded wire, leaving the desired length for the force sensor resistor. Connect FSR pin 1 to +5V. Connect FSR pin 2 to A0 on board. Connect 10 KOhm resistor to GND and FSR pin 2 (Figure 13).
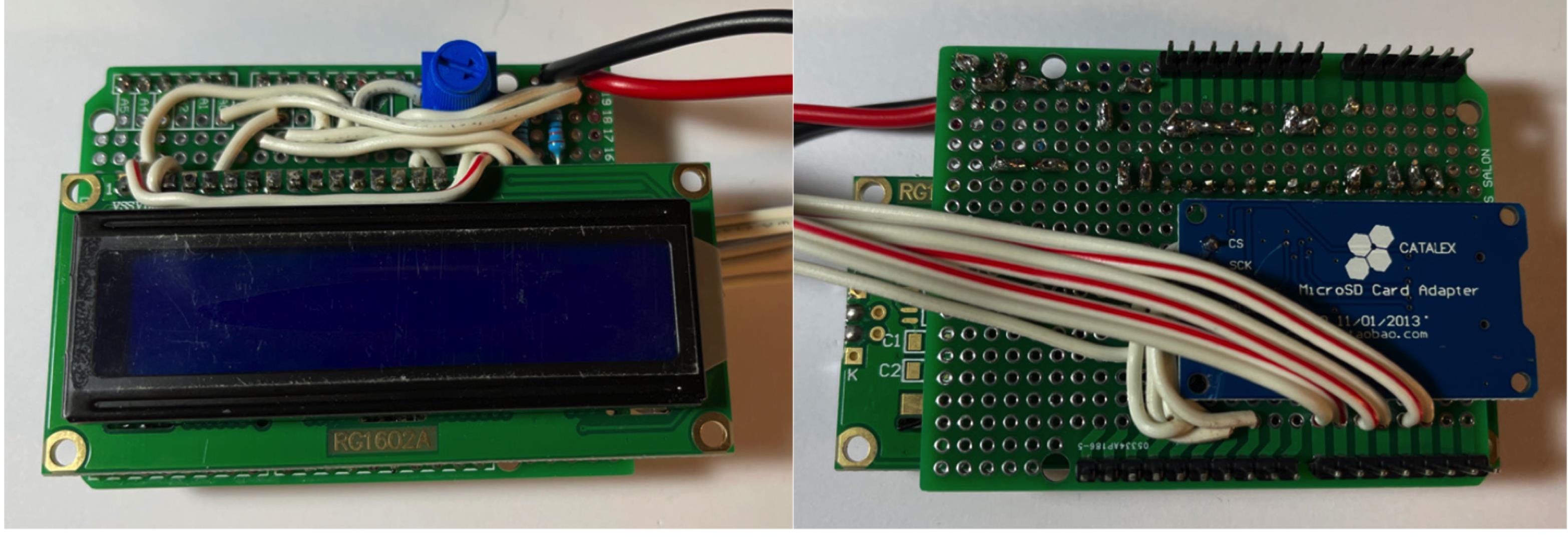
Figure 13. Connect FSR wires.Wrap the longer wires from steps 3 & 4 around to the LCD and connect as follows: Micro SD card adapter VCC to +5V, board D2 to LCD D7, board D3 to LCD D6, board D4 to LCD D5, board D5 to LCD D4, board D6 to LCD E, and board D7 to LCD RS (Figure 14).
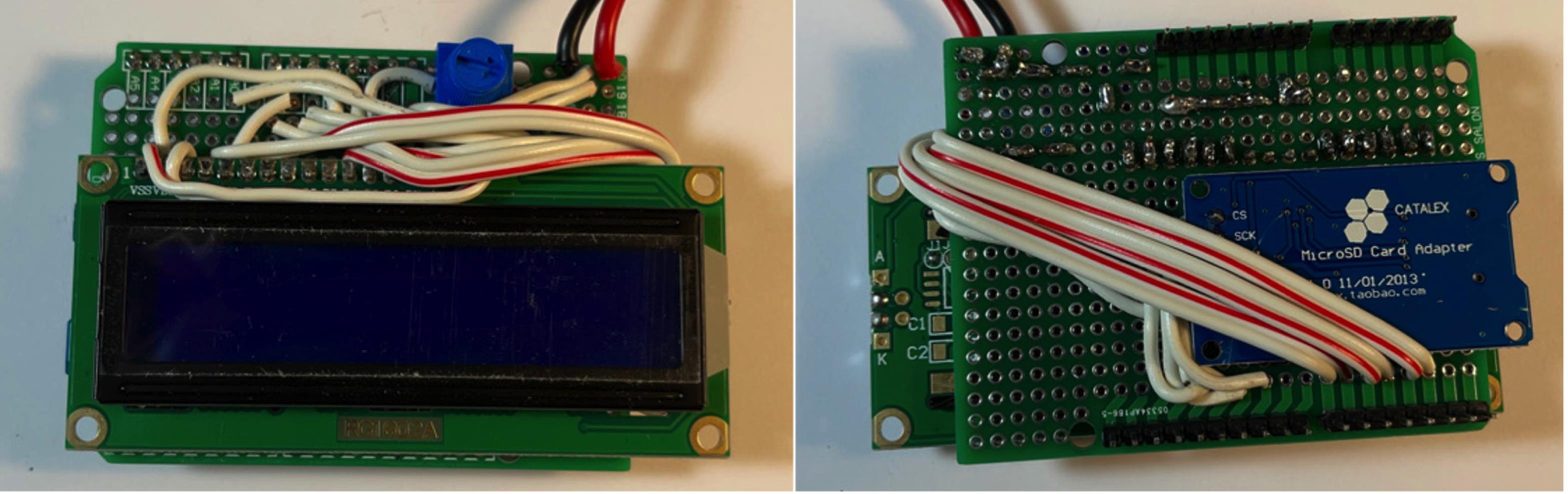
Figure 14. Connect digital outputs to LCD screen.Connect stranded wire to FSR, and reinforce with electrical tape (Figure 15).
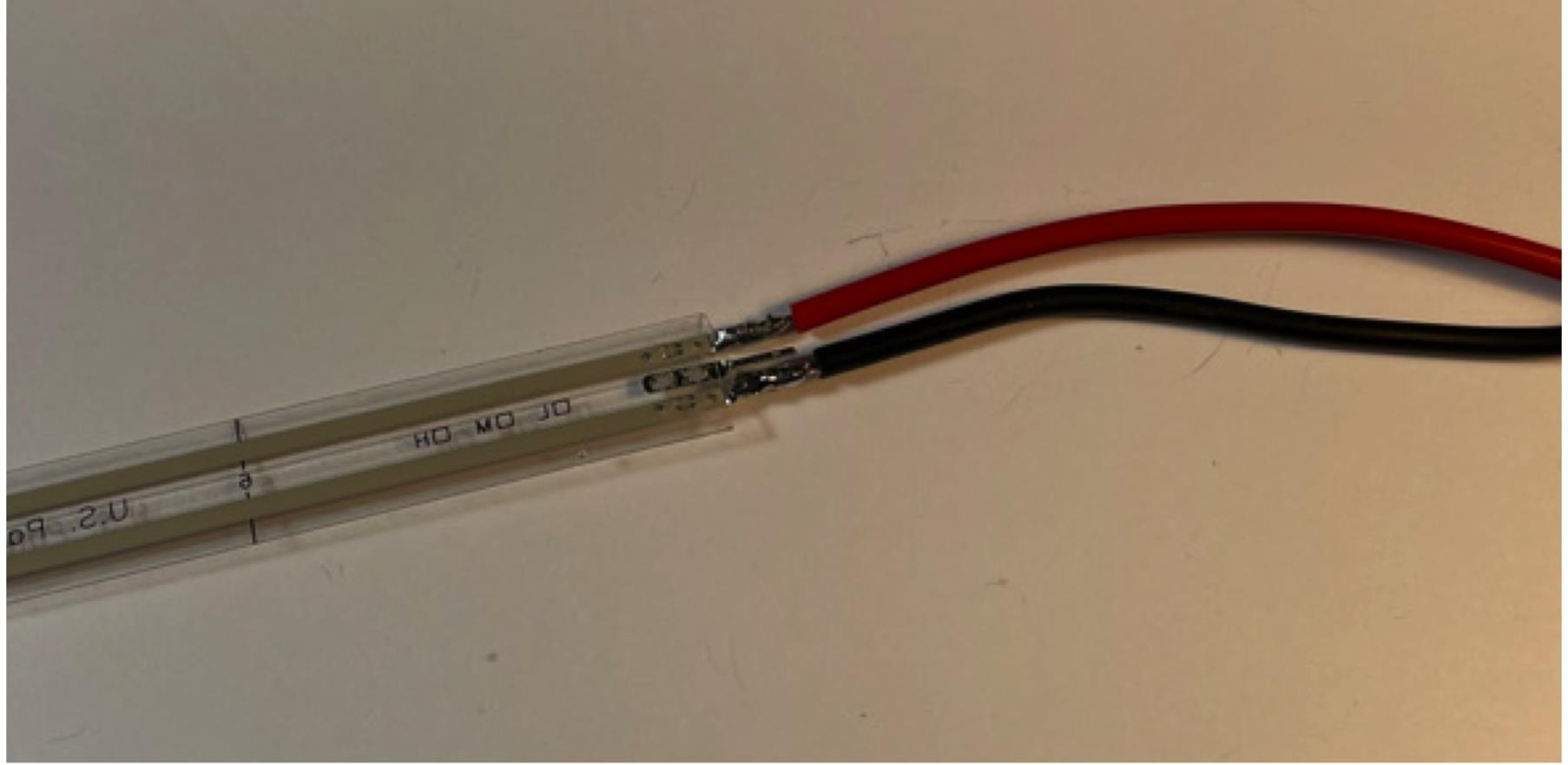
Figure 15. Connect FSR.Connect toggle switch to 9V battery clip in series. Connect 9V battery.
Insert Micro SD card into Micro SD card adapter.
Attach Arduino Uno by fitting the male pins of your device into the female connecters on the Arduino. Plug 9V battery power into the Arduino.
Use a computer-aided design (CAD) to make a housing unit for the device. We recommend Tinkercad or Slidworks. Print unit with 3D printer. Our housing design is available at https://github.com/gwandling/PrecisionPinch. Device construction is complete (Figure 16).

Figure 16. Completed pressure sensor device with portable housing unit.
Programming and Calibration for the Pressure Sensor
Download the open source Arduino software from the company website.
Download the PrecisionPinch Software from https://github.com/gwandling/PrecisionPinch.
Plug in Arduino to your computer and upload the PrecisionPinch software to the device.
Calibrate the device by plotting the measured resistance against a known pressure by using known weights. We recommend a weight series from 2.5 to 25 lbs. Be sure to use Force = Pressure/Area in consideration of your calculations. Next, convert resistance to conductance. Use the inverse of this plot to formulate a calibration equation. An example calibration file can be found at https://github.com/gwandling/PrecisionPinch.
Correct the calibration equation in the PrecisionPinch software with your own calibration equation.
Upload your modified software to the device.
The device is now ready for use.
Data Retrieval for Pressure Sensor
With the use of a computer, data can be retrieved after device use from the micro SD card under the file DATALOG.txt. This file can be uploaded to Microsoft Excel for further analysis. An example is shown in Figure 17.
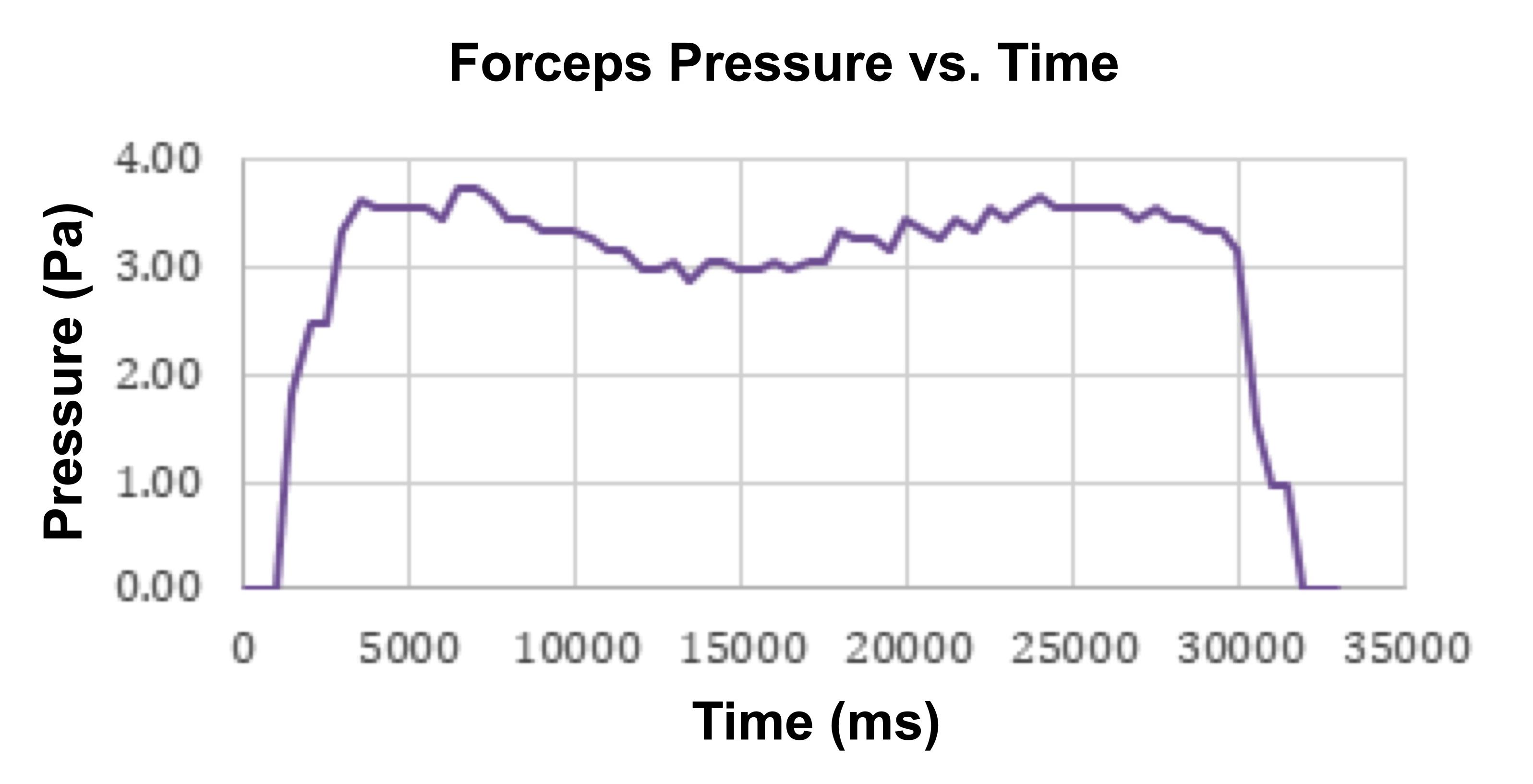
Figure 17. Example of PrecisionPinch data. A representative tracing showing the time-course for crush injury pressure generated by a forceps and graphed in Excel. Pa, pascals; ms, millisecond.
Data analysis
For Pressure Sensor
Data analysis can be performed in Microsoft Excel, where graphs of time vs. pressure can be created to visualize the change in pressure during crush injury. The average pressure can be calculated to compare different models of crush injuries.
Notes
For Stepwise Transection with fibrin Glue
Complete transection of the nerve after the second cut should be confirmed under the operating microscope. If the nerve is not completely transected, this could a confounding factor in outcome interpretation.
Do not free the sciatic nerve from the surrounding connective tissue. If the nerve was isolated from the surrounding connective tissue, the severed nerve ends could be retracted more after transection.
Do not stretch the hindlimb during the entire procedure, as stretching the leg could induce gap formation between the severed nerve ends before fibrin glue clotting.
Always keep an appropriate control for STG, such as simple one-touch complete transection of the sciatic nerve with glue, as described in a recent publication (Lee et al., 2020).
The device is designed to sense and record most moderate amounts of pressure applied to it, with a response time of <5 µsec and pressure range of 2.5–25 lbs, but it does not sense a simple contact or touch.
For durability, the sensor is rated for 3 million actuations. However, like all measuring devices, it should be recalibrated every couple of months to ensure accurate readings.
We validated different forceps and needle drivers for the reproducibility of the pressure they produced when pressed and held in a fixed position. The sequential steps to press the nerve over the sensor and capture the pressure signals are described in detail in our recent publication (Wandling et al., 2021).
Please note that the pressure is only measured within the black circle of the sensor. Confirm that all desired contact is within that dimension.
Acknowledgments
The novel nerve transection protocol is adapted from the original version of our article (Lee et al., 2020) and the pressure sensor construction is presented in more details than our original report (Wandling et al., 2021). This work was supported by grants from the NIH (K08 AR060164-01A), DOD (W81XWH-16-1-0725), and National Research Foundation of Korea (NRF-2015R1C1A1A02036830) in addition to institutional support from the Pennsylvania State University Medical Center. The authors are thankful to Mosammat Begom, MS, for technical help.
Competing interests
John C. Elfar, M.D. has equity interest in a company that has licensed patents from his institution based on his work.
Ethics
The experimental design and animal protocol (PRAMS201747923, 11/17/2020-11/17/2023) was approved and renewed by the Institutional Animal Care and Use Committee at Penn State University College of Medicine, Hershey, Pennsylvania, USA.
References
Alant, J. D., Kemp, S. W., Khu, K. J., Kumar, R., Webb, A. A. and Midha, R. (2012). Traumatic neuroma in continuity injury model in rodents. J Neurotrauma 29(8): 1691-1703.
Bridge, P. M., Ball, D. J., Mackinnon, S. E., Nakao, Y., Brandt, K., Hunter, D. A. and Hertl, C. (1994). Nerve crush injuries--a model for axonotmesis. Exp Neurol 127(2): 284-290.
Caillaud, M., Richard, L., Vallat, J. M., Desmouliere, A. and Billet, F. (2019). Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res 14(1): 24-33.
Campbell, W. W. (2008). Evaluation and management of peripheral nerve injury. Clin Neurophysiol 119(9): 1951-1965.
Chen, L. E., Seaber, A. V., Glisson, R. R., Davies, H., Murrell, G. A., Anthony, D. C. and Urbaniak, J. R. (1992). The functional recovery of peripheral nerves following defined acute crush injuries. J Orthop Res 10(5): 657-664.
Faroni, A., Mobasseri, S. A., Kingham, P. J. and Reid, A. J. (2015). Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 82-83: 160-167.
Foster, C. H., Karsy, M., Jensen, M. R., Guan, J., Eli, I. and Mahan, M. A. (2019). Trends and Cost-Analysis of Lower Extremity Nerve Injury Using the National Inpatient Sample. Neurosurgery 85(2): 250-256.
Geary, M. B., Li, H., Zingman, A., Ketz, J., Zuscik, M., De Mesy Bentley, K. L., Noble, M. and Elfar, J. C. (2017). Erythropoietin accelerates functional recovery after moderate sciatic nerve crush injury. Muscle Nerve 56(1): 143-151.
Hare, G. M., Evans, P. J., Mackinnon, S. E., Best, T. J., Bain, J. R., Szalai, J. P. and Hunter, D. A. (1992). Walking track analysis: along-term assessment of peripheral nerve recovery. Plast Reconstr Surg 89(2): 251-258.
Isaacs, J. (2013). Major peripheral nerve injuries. Hand Clin 29(3): 371-382.
Karsy, M., Watkins, R., Jensen, M. R., Guan, J., Brock, A. A. and Mahan, M. A. (2019). Trends and Cost Analysis of Upper Extremity Nerve Injury Using the National (Nationwide) Inpatient Sample. World Neurosurg 123: e488-e500.
Lee, J. I., Gurjar, A. A., Talukder, M. A. H., Rodenhouse, A., Manto, K., O'Brien, M., Govindappa, P. K. and Elfar, J. C. (2020). A novel nerve transection and repair method in mice: histomorphometric analysis of nerves, blood vessels, and muscles with functional recovery. Sci Rep 10(1): 21637.
Moore, A. M., Borschel, G. H., Santosa, K. B., Flagg, E. R., Tong, A. Y., Kasukurthi, R., Newton, P., Yan, Y., Hunter, D. A., Johnson, P. J., et al. (2012). A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J Neurosci Methods 204(1): 19-27.
Noble, J., Munro, C. A., Prasad, V. S. and Midha, R. (1998). Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45(1): 116-122.
Robinson, L. R. (2000). Traumatic injury to peripheral nerves. Muscle Nerve 23(6): 863-873.
Robinson, L. R. (2004). Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol 57: 173-186.
Savastano, L. E., Laurito, S. R., Fitt, M. R., Rasmussen, J. A., Gonzalez Polo, V. and Patterson, S. I. (2014). Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J Neurosci Methods 227: 166-180.
Siemionow, M. and Brzezicki, G. (2009). Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol 87: 141-172.
Tos, P., Battiston, B., Ciclamini, D., Geuna, S. and Artiaco, S. (2012). Primary repair of crush nerve injuries by means of biological tubulization with muscle-vein-combined grafts. Microsurgery 32(5): 358-363.
Tseng, K. C., Li, H., Clark, A., Sundem, L., Zuscik, M., Noble, M. and Elfar, J. (2016). 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol Med 8(12): 1409-1420.
Wandling, G. D., Lee, J. I., Talukder, M. A. H., Govindappa, P. K. and Elfar, J. C. (2021). Novel Real-time DigitalPressure Sensor Reveals Wide Variations in Current Nerve Crush Injury Models. Mil Med 186(Suppl 1): 473-478.
Yue, L., Talukder, M. A. H., Gurjar, A., Lee, J. I., Noble, M., Dirksen, R. T., Chakkalakal, J. and Elfar, J. C. (2019). 4-Aminopyridine attenuates muscle atrophy after sciatic nerve crush injury in mice. Muscle Nerve 60(2): 192-201.
Zochodne, D. W. (2012). The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst 17(1): 1-18.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lee, J. I., Wandling, G. D., Talukder, M. A. H., Govindappa, P. K. and Elfar, J. C. (2022). A Novel Standardized Peripheral Nerve Transection Method and a Novel Digital Pressure Sensor Device Construction for Peripheral Nerve Crush Injury. Bio-protocol 12(5): e4350. DOI: 10.21769/BioProtoc.4350.
Category
Neuroscience > Peripheral nervous system > Sciatic nerve
Neuroscience > Nervous system disorders
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








