- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Development of a 3D Bioprinted Scaffold with Spatio-temporally Defined Patterns of BMP-2 and VEGF for the Regeneration of Large Bone Defects
Published: Vol 11, Iss 21, Nov 5, 2021 DOI: 10.21769/BioProtoc.4219 Views: 3416
Reviewed by: Giusy TornilloJaira Ferreira de VasconcellosAnonymous reviewer(s)
Abstract
The local delivery of growth factors such as BMP-2 is a well-established strategy for the repair of bone defects. The limitations of such approaches clinically are well documented and can be linked to the need for supraphysiological doses and poor spatio-temporal control of growth factor release in vivo. Using bioprinting techniques, it is possible to generate implants that can deliver cytokines or growth factors with distinct spatiotemporal release profiles and patterns to enhance bone regeneration. Specifically, for bone healing, several growth factors, including vascular endothelial growth factor (VEGF) and bone morphogenic proteins (BMPs), have been shown to be expressed at different phases of the process. This protocol aims to outline how to use bioprinting strategies to deliver growth factors, both alone or in combination, to the site of injury at physiologically relevant dosages such that repair is induced without adverse effects. Here we describe: the printing parameters to generate the polymer mechanical backbone; instructions to generate the different bioinks and allow for the temporal control of both growth factors; and the printing process to develop implants with spatially defined patterns of growth factors for bone regeneration. The novelty of this protocol is the use of multiple-tool fabrication techniques to develop an implant with spatio-temporal control of growth factor delivery for bone regeneration. While the overall aim of this protocol was to develop an implant for bone regeneration, the technique can be modified and used for a variety of regenerative purposes.
Graphic abstract:
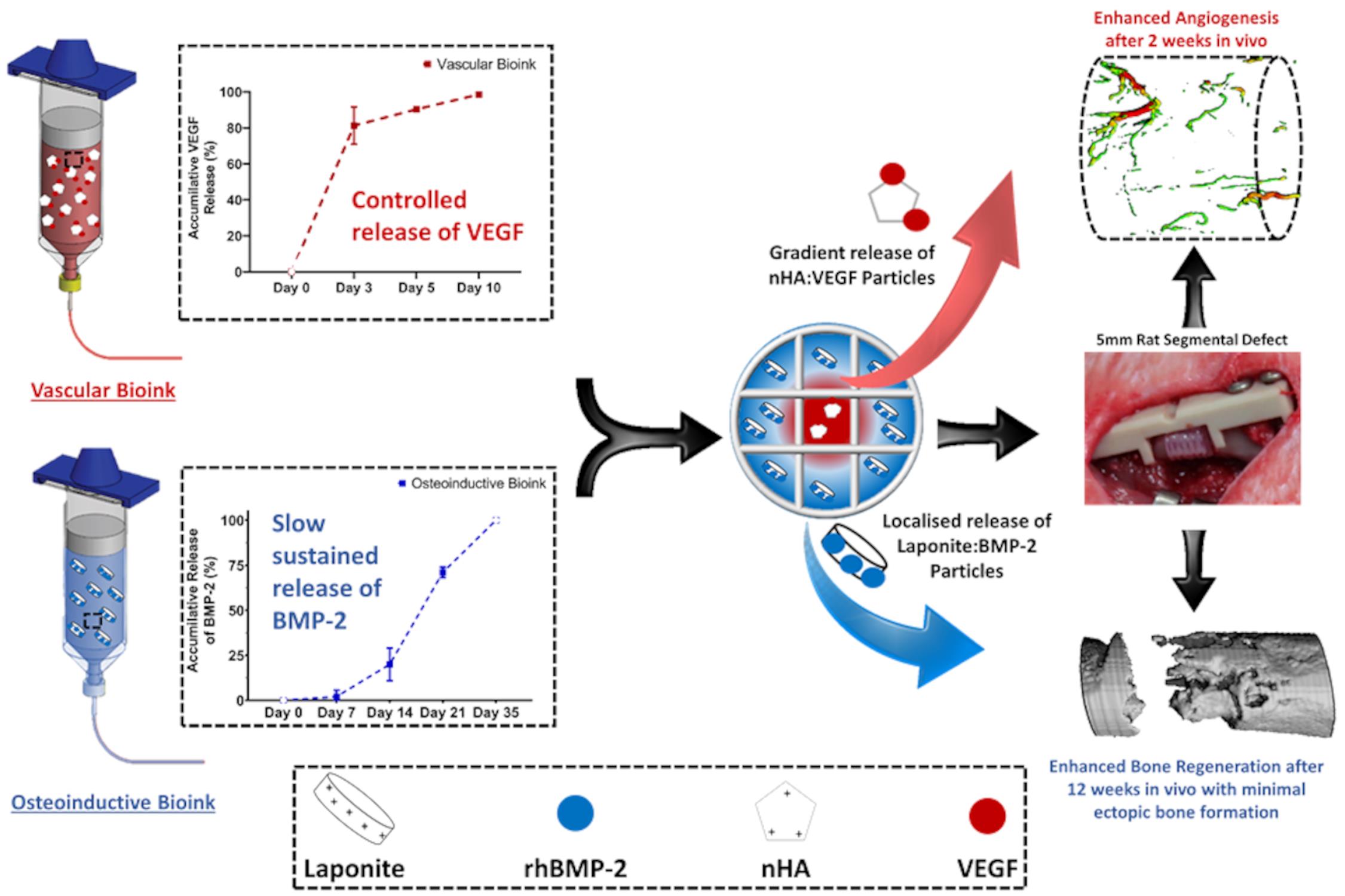
3D Bioprinting Spatio-Temporally Defined Patterns of Growth Factors to Tightly Control Bone Tissue Regeneration.
Background
The limitations of local delivery of growth factors clinically are well documented and can be linked to the need for supraphysiological doses. Multiple-tool biofabrication techniques overcome this limitation by making it is possible to generate ‘print-and-implant’ devices to deliver growth factors with distinct spatiotemporal release profiles to enhance tissue regeneration. Specifically, for bone healing, several growth factors have been shown to be expressed at different phases of healing, including vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs). This protocol describes in detail how we developed a bioinks that interact with the growth factors to make it possible to tightly control the location and timing of their release in vivo, thereby negating the need for supraphysiological doses.
Materials and Reagents
Luer lock syringe cap seal (Adhesive Dispensing, catalog number: 7015LLPK)
10cc clear syringe barrel (Adhesive Dispensing, catalog number: 7100LL1NPK)
10cc syringe piston (Adhesive Dispensing, catalog number: 7100009WPK)
27 G needle, 0.25'' long (Adhesive Dispensing, catalog number: TE727025PK)
5 ml syringe (VWR, catalog number: 613-2043)
RGD γ-irradiated alginate (Manufactured in house by collaborators in Alsberg’s Lab)
Commercially available NOVATACHTM peptide-coupled alginate could also be used, but the crosslinking ratios would need to be modified depending on the MW of the alginate used.
Sodium Triphosphate (Sigma-Aldrich, catalog number: 72061)
Darvan 821AVR (Vanderbilt Minerals, DARVAN® 821-A)
Sodium hydroxide (Sigma-Aldrich, catalog number: S8045)
Vascular Endothelial Growth Factor (Peprotech, catalog number: 100-20)
Bone Morphogenic Protein-2 (Peprotech, catalog number: 120-02)
Methylcellulose (Sigma-Aldrich, catalog number: M7027)
Dulbecco’s modified Eagle medium (GlutaMAXTM; ThermoFisher, catalog number: 61965026)
Fetal bovine serum (FBS; Thermo Scientific, catalog number: 10270-106)
Penicillin streptomycin (Sigma-Aldrich, catalog number: 15070063)
Laponite (Laponite XLG, BYK Additives & Instruments)
Polycaprolactone (PCL; CAPA 6500D, Perstorp, Mn = 50 kDa)
Calcium Chloride (Sigma-Aldrich, catalog number: C5670)
Calcium sulfate (Sigma-Aldrich, catalog number: 255696)
3.5% RGD γ-irradiated alginate solution (see Recipes)
100 mM calcium chloride solution (see Recipes)
60 mM calcium sulphate solution (see Recipes)
Sodium triphosphate solution (see Recipes)
Equipment
Sterile stirrer bar and stirrer plate
3D bioplotter (RegenHU, 3D Discovery, Generation 1)
Software
BioCAD (RegenHU, 3D Discovery, Generation 1)
GraphPad (GraphPad Software, La Jolla, CA, USA; www.graphpad.com)
Procedure
3D printing process for PCL backbone
Use a 3D bioplotter from RegenHU to print all the scaffolds.
Print constructs of 4 mm in diameter and 5 mm in height, with both lateral and horizontal porosity and a fibre spacing of 1.2 mm, with polycaprolactone using a 30G needle, Figure 1.
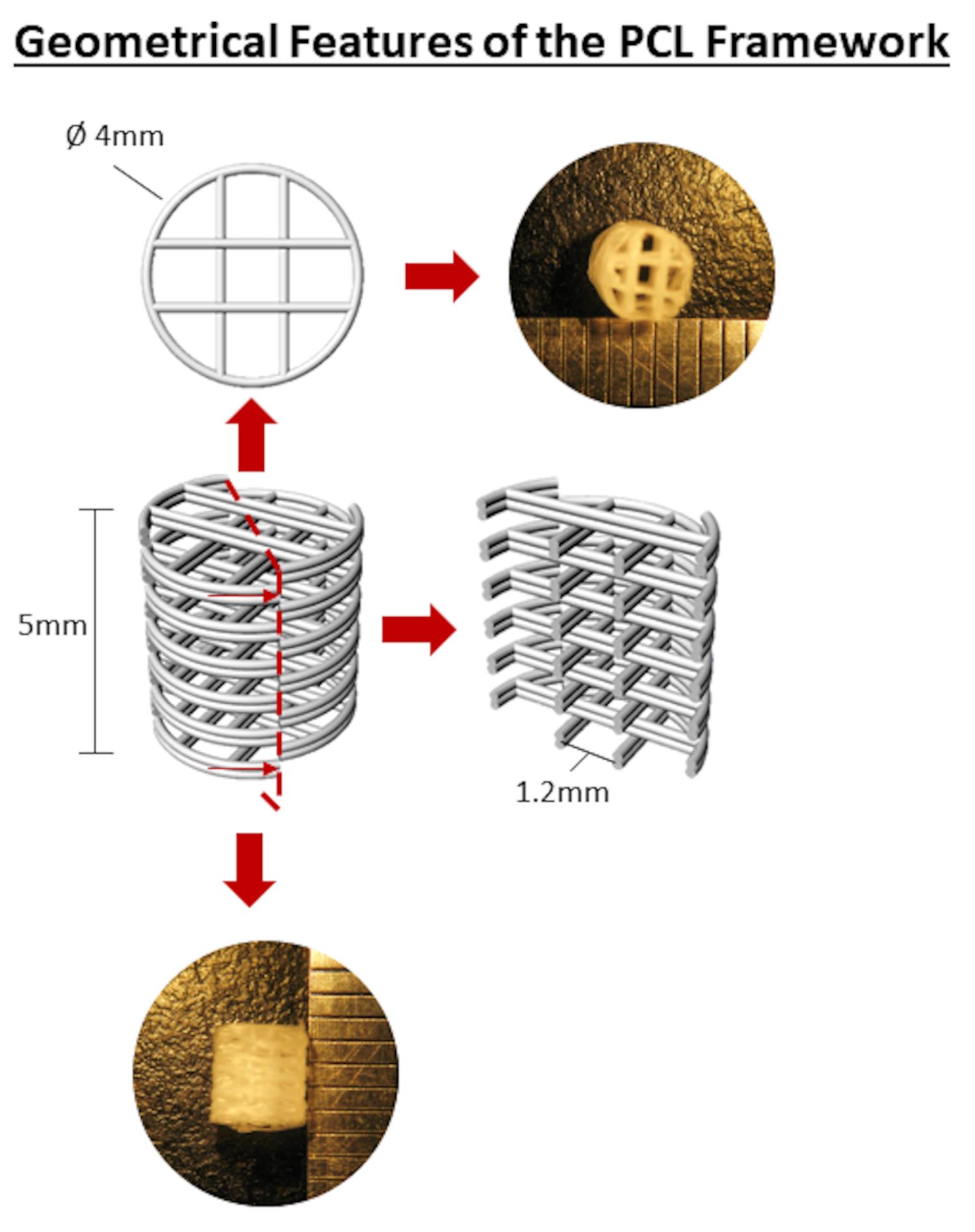
Figure 1. Geometrical Features of the PCL FrameworkThe printing parameters of the PCL are listed in Table 1.
Sterilise the scaffolds using ethylene oxide (ETO) sterilisation prior to hydrogel printing.
Table 1. Parameters for printing the scaffolds with PCL using the RegenHU bioprinter
Heat of the thermopolymer Tank 69°C Heat of the thermopolymer Head 72°C Pressure 1 bar Screw Speed 30 rev/min Feed Rate (Translational Speed) 3 mm/s
Generation of vascular and osteoinductive bioinks
Note: Each solution should be freshly made just prior to printing.
Prepare the 3.5% RGD γ-irradiated alginate solution (see Recipes).
Add methylcellulose (2:1) (w/w) particles and mix into solution using a sterile stirrer bar and stirrer plate. For 5 g of alginate, add 2.5 g of sterile methylcellulose.
Prepare nHA in sterile conditions following a previously described protocol (Cunniffe et al., 2010). Briefly:
Prepare the 100mM calcium chloride solution (see Recipes).
Prepare the sodium triphosphate solution (see Recipes).
Once fully dissolved, filter sterile both solutions using a filter with a pore size of 0.45 µm.
Slowly add the sodium triphosphate solution to the 100 mM calcium chloride solution and leave stirring for 2 min.
Centrifuge solution at maximum speed for 60 min.
Pour off supernatant and weigh pellet. Add the appropriate amount of nHA wet weight to bioink.
Add either nHA (2:1:2) (for vascular bioink) or Laponite (6:3:1) (for osteoinductive bioink) (w/w) to the alginate methylcellulose blend and mix into solution using a sterile stirrer bar and stirrer plate for 2-3 h. For example, for every 5 g of alginate, add 5 g of nHA or 0.83 g of Laponite.
Using a dual syringe approach linked via a luer lock, load bioinks with either VEGF (100 ng/ml) or BMP-2 (10 µg/ml) and pre-crosslink with the 60 mM calcium sulphate solution at a v/v ratio of 7:3 (see Figure 2). Allow bioinks to crosslink for 30 min before being printing.
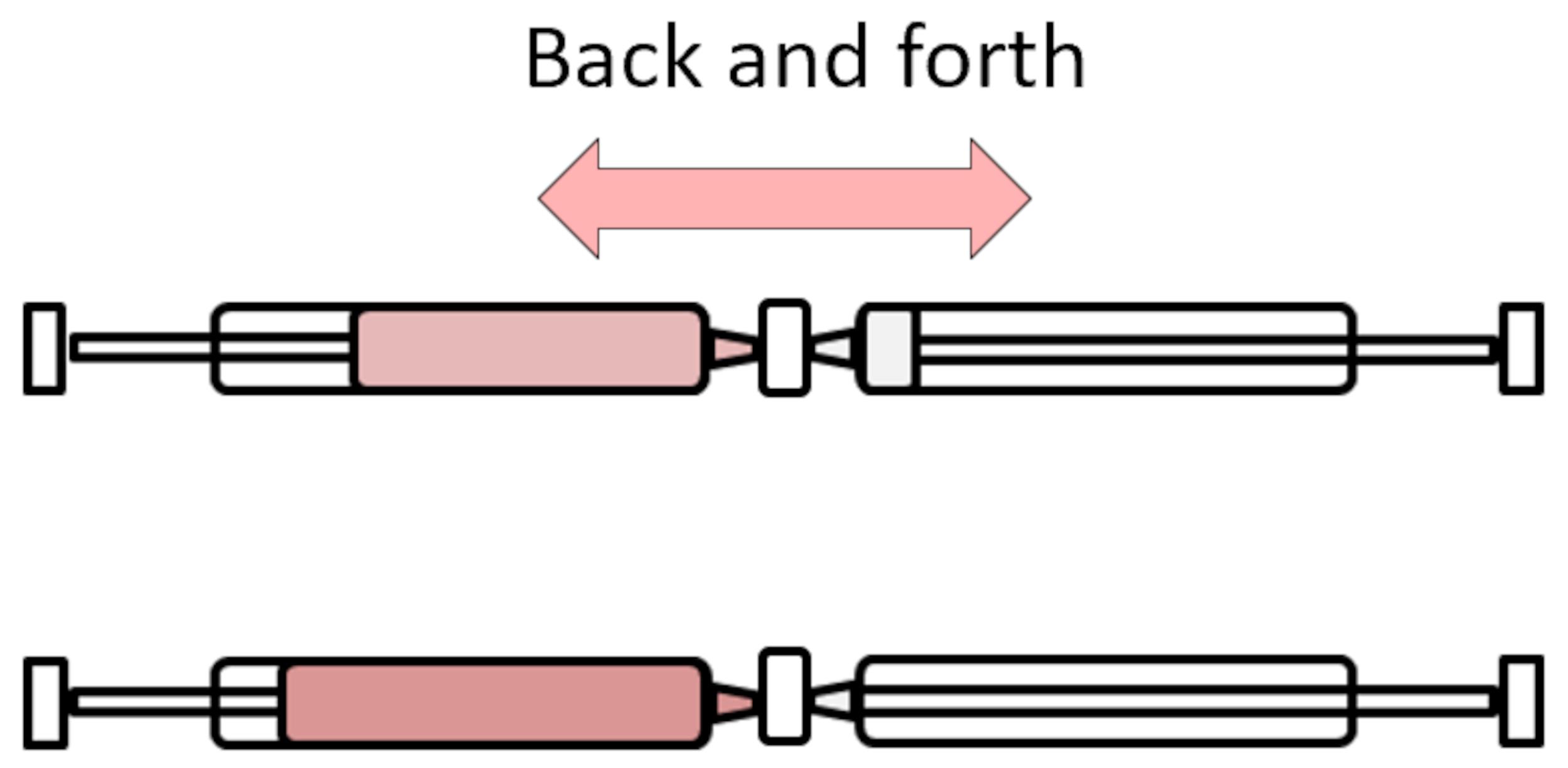
Figure 2. Schematic of dual-syringe crosslinking approach
3D printing process of the implants
Place the piston in the 5cc barrel and load each bioink, either vascular or osteoinductive, into the piston using the luer lock.
Place each barrel in the two hydrogel heads of the 3D printer.
The printing parameters of the PCL are listed in Table 2.
Table 2. Printing parameters for printing the bioinks within the PCL framework using the RegenHU bioprinter
Pressure 1 bar Needle Size 27 G Feed Rate (Translational Speed) 3 mm/s Via manipulation of the G-code (see Supplementary file) of the printer, z-stack each hydrogel at room temperature into the PCL framework to generate the following experimental groups: (1) VEGF Gradient, the vascular bioink loaded with 500 ng/ml of VEGF in the centre of the implant and base bioink in the periphery; (2) BMP-2 gradient, the osteoinductive bioink loaded with 10 µg/ml BMP-2 in the implant periphery (2 µg/construct), with the base bioink in the centre; (3) Composite (VEGF+BMP-2), the osteoinductive bioink in the periphery with the vascular bioink in the centre (see Figure 3A in Freeman et al., 2020), see Figure 3.
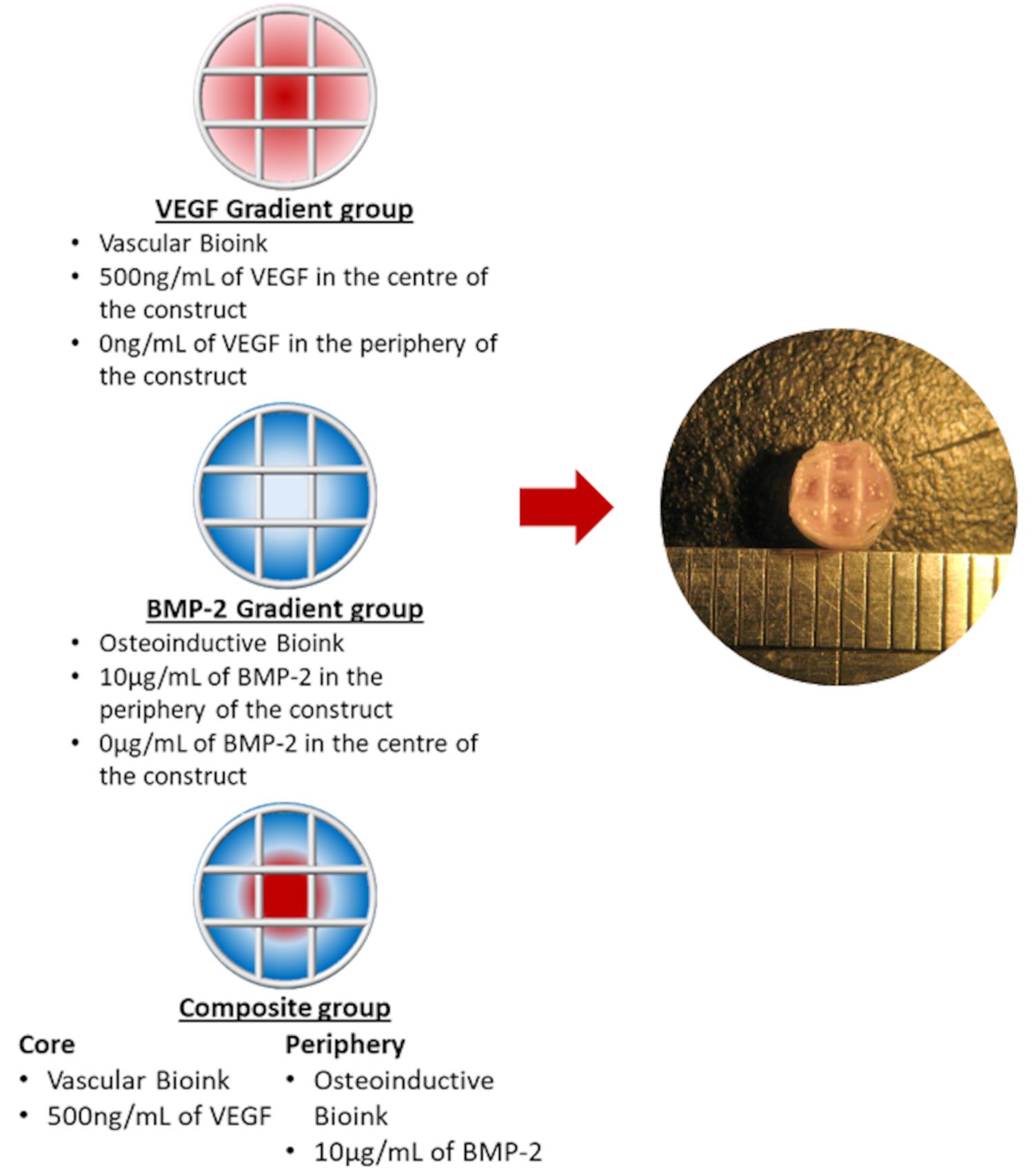
Figure 3. Schematic of the bioink pattern for each group and Marco image of the final implantCrosslink post-printing constructs again in a bath Petri-dish filled with the 100 mM calcium chloride solution using a sterile tweezer and allowed to further crosslink for 1 min.
Constructs must be used immediately; if being implanted, the constructs should be printed the morning of surgeries.
Data analysis
Description of all in vivo analyses can be found in the original publication (Freeman et al., 2020). Statistics were performed using the following variables: (i) when there were two groups and one time point, a standard two-tailed t-test was performed; (ii) when there were more than two groups and one time point, a one-way analysis of variance (ANOVA) was performed; and (iii) when there were more than two groups and multiple time points, a two-way ANOVA was performed. All analyses were performed using GraphPad. For all comparisons, the level of significance was P ≤ 0.05.
Recipes
3.5% RGD γ-irradiated alginate solution
Dissolve the RGD γ-irradiated alginate in growth medium consisting of high-glucose Dulbecco’s modified Eagle medium (DMEM), 10% fetal bovine serum, 100 U/ml penicillin, and 100 g/ml streptomycin to make up a final concentration of 3.5% (w/v) alginate.
100 mM calcium chloride solution
Mix 0.735 g of CaCl2 into 50 ml of sterile water.
Vortex and place on a rotator to dissolve.
60 mM calcium sulphate solution
Mix 0.408g of CaSO4 into 50 ml of sterile water (note that this solution does not fully dissolve and needs to be mixed directly before use each time).
Sodium triphosphate solution
Mix 1.135 mg Na3PO4·12H2O, 10 mg of NaOH pellets, and 100 µl of Darvan into 50 ml of sterile ultra-pure water.
Acknowledgments
This publication has emanated from research supported by a research grant from European Research Council (ERC) under Grant Number 647004 and the Irish Research Council (GOIPD/2016/324). The protocol was adapted from the previous publication “3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration.” Published in Science Advances 2020 (Freeman et al., 2020).
Competing interests
There are no conflicts of interest or competing interests.
References
- Cunniffe, G. M., O'Brien, F. J., Partap, S., Levingstone, T. J., Stanton, K. T. and Dickson, G. R. (2010). The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J Biomed Mater Res A 95(4): 1142-1149.
- Freeman, F. E., Pitacco, P., van Dommelen, L. H. A., Nulty, J., Browe, D. C., Shin, J. Y., Alsberg, E. and Kelly, D. J. (2020). 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv 6(33): eabb5093.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Freeman, F. E., Pitacco, P., van Dommelen, L. H. A., Nulty, J., Browe, D. C., Shin, J. Y., Alsberg, E. and Kelly, D. J. (2021). Development of a 3D Bioprinted Scaffold with Spatio-temporally Defined Patterns of BMP-2 and VEGF for the Regeneration of Large Bone Defects. Bio-protocol 11(21): e4219. DOI: 10.21769/BioProtoc.4219.
- Freeman, F. E., Pitacco, P., van Dommelen, L. H. A., Nulty, J., Browe, D. C., Shin, J. Y., Alsberg, E. and Kelly, D. J. (2020). 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv 6(33): eabb5093.
Category
Biological Engineering > Bioprinting
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











