- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Whole-mount Staining of Mouse Diaphragm Neuromuscular Junctions
(*contributed equally to this work) Published: Vol 11, Iss 21, Nov 5, 2021 DOI: 10.21769/BioProtoc.4215 Views: 4619
Reviewed by: Giusy TornilloKae-Jiun ChangVictor Steven Van LaarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantifying Intracellular Distributions of HaloTag-Labeled Proteins With SDS-PAGE and Epifluorescence Microscopy
Julia Shangguan and Ronald S. Rock
Jul 20, 2025 2501 Views

Fluorescence Lifetime-Based Separation of FAST-Labeled Cellular Compartment
Aidar R. Gilvanov [...] Yulia A. Bogdanova
Oct 5, 2025 1323 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views
Abstract
The neuromuscular junction (NMJ) is a specialized synapse that connects the terminal end of a motor neuron and a skeletal muscle fiber. Defects in NMJ cause abnormalities of neuromuscular transmission, leading to NMJ disorders. The mammalian diaphragm muscle is essential for respiration and has been widely used to study NMJ formation. Here, we provide a simple and straightforward protocol for preparing diaphragms from embryonic, neonatal, and adult mice and for subsequent NMJ staining.
Keywords: Whole-mount stainingBackground
The mammalian diaphragm separates the thoracic cavity from the abdominal cavity. It is composed of two parts: the costal and crural diaphragm segments (Merrell and Kardon, 2013). neuromuscular junction (NMJ) is a specialized structure on the diaphragm, consisting of presynaptic nerve terminals and postsynaptic muscle membranes (Li et al., 2018). With the development of the mouse embryos, NMJ gradually gathers in the middle area of the diaphragm myofibers. In adult mice, each contractile diaphragm myofiber is precisely dominated by a motor neuron terminal at the middle region (Liu and Chakkalakal, 2018). When motoneuron terminals release acetylcholine (ACh), the latter activates its receptors (AChRs) located on muscle fibers and causes muscle contraction (Li et al., 2018). Defects in the development and maintenance of NMJ can lead to a variety of neuromuscular diseases (Arimura et al., 2014). Because of the particularity of its structure, diaphragms are frequently used to study NMJ formation. The embryonic and neonatal mouse diaphragms are thin and easy to stain. However, adult diaphragms become thicker and are covered with connective tissues, and are difficult for immunostaining. In this article, we provide a simple and detailed protocol that can be used for the isolation of diaphragms from embryonic, neonatal, and adult mice, and for diaphragm NMJ staining.
Materials and Reagents
60 mm tissue culture dish (Shanghai Sunub Bio-Tech Development Inc, catalog number: TCD-60)
15 ml centrifuge tube (Corning Incorporated-Life Sciences, catalog number: 430791)
50 ml centrifuge tube (Corning Incorporated-Life Sciences, catalog number: 430829)
12-well culture cluster (Corning Incorporated-Life Sciences, catalog number: CLS3512-100EA)
25 × 75 mm adhesion microscope slides (CITOTEST, CITOTEST®, catalog number: 188105)
24 × 50 mm microscope cover glass (Titan, catalog number: 02036401)
Foam board (15 × 10 × 4 cm)
Needles (Shanghai Kindly Group, description: 0.45 mm × 16 mm RW LB)
Aluminum foil (Top group, Miaojie, catalog number: MF50)
Black incubator box (Haimen Tech Experiment equipment, catalog number: G1050)
Mice: Mice strain: C57BL/6J
Mouse embryos (at embryonic days, E16.5)
Neonatal pups (at the postnatal day 1, P1)
Adult mice (2-month-old, 2M)
Triton X-100 (Shanghai Shisheng Sibas Technology Development Co.,Ltd, catalog number: H2Q0212)
Na2HPO4·12H2O (Shanghai Experiment Reagent Co.,Ltd, catalog number: 174710)
KH2PO4 (Shanghai Experiment Reagent Co.,Ltd, catalog number: 168130)
NaCl (Sinopharm Chemical Reagent Co.,Ltd, catalog number: 10019318)
KCl (Sinopharm Chemical Reagent Co.,Ltd, catalog number: 10016318)
Liquid blocker super pap pen (Daido Sangyo Co.,Ltd, catalog number: Daido-Sangyo.SBM05480)
Normal goat serum (Jackson ImmunoResearch Laboratories Inc., catalog number: 005-000-121)
Primary antibody anti-synaptophysin (rabbit) (Thermo Fisher Scientific, catalog number: PA1-1043)
α-Bungarotoxin-ATTO Fluor-488 (Alomone Labs, catalog number: B-100-AG)
Secondary antibody Alexa FluorTM 546 goat anti-rabbit IgG (Thermo Fisher Scientific, catalog number: A-11035)
FluoromountTM Aqueous Mounting Medium (Sigma-Aldrich, catalog number: F4680-25ML)
10× PBS (see Recipes)
4% paraformaldehyde (PFA) (see Recipes)
0.5% Triton X-100 in PBS (see Recipes)
0.1 M glycine (see Recipes)
Blocking solution (see Recipes)
Primary antibody solution (see Recipes)
Secondary antibody solution (see Recipes)
Equipment
Curved tip eye forceps (Shanghai Shisheng Sibas Technology Development Co.,Ltd, catalog number: D020102)
Ophthalmic scissors (Shanghai Shisheng Sibas Technology Development Co.,Ltd, catalog number: D010101)
Straight forceps (Shanghai Shisheng Sibas Technology Development Co.,Ltd, catalog number: 433050)
Arrow forceps (Shanghai Shisheng Sibas Technology Development Co.,Ltd, catalog number: 433051)
Zeiss Confocal Laser-scanning Microscope (Zeiss, model: LSM880NLO FLIM)
Software
Procedure
Diaphragm separation
Embryonic diaphragm (E16.5) separation (Video 1)
At the E16.5 stage, euthanize pregnant mouse by cervical dislocation.
Spray 75% ethanol on the mouse and place the mouse facing up.
Use the ophthalmic scissors to quickly cut the skin and muscle along the midline, then take out the embryos.
Fix whole embryos with 4% PFA overnight at 4°C.
Put the embryo in PBS, and cut along the ribs to remove the lower body. Then cut along the armpit to remove the upper body.
Separate the lungs, diaphragm, and liver together from the chest cavity. Remove the lungs and livers carefully. This step ensures the integrity of the diaphragm.
Video 1. Separation of the diaphragm from a mouse embryoNeonatal diaphragm (P1) separation (Video 2)
Euthanize pup at the P1 by carbon dioxide inhalation and decapitation.
Cut along the ribs to remove the lower body.
Use the ophthalmic scissors to cut a small opening at the junction of the diaphragm and the chest cavity.
Carefully separate the diaphragm with curved tip eye forceps and straight forceps.
Put the diaphragm in PBS and flatten it.
Remove other tissues from the diaphragm.
Fix the diaphragm with 4% PFA.
Video 2. Separation of the diaphragm from a neonatal pupAdult diaphragm (2M) separation (Video 3)
Euthanize an adult mouse by cervical dislocation.
Spray 75% ethanol on the mouse and pin the mouse facing up.
Use the ophthalmic scissors to quickly cut the skin and muscle along the midline.
Separate the other tissues from the diaphragm.
Clamp the edge of the diaphragm and cut along it with the ophthalmic scissors to separate it from other tissues.
Put the diaphragm in PBS and flatten it.
Remove the other tissues from the diaphragm.
Fix the diaphragm with 4% PFA.
Video 3. Separation of the diaphragm from an adult mouse
Immunofluorescence staining of diaphragms
Embryonic/neonatal diaphragm staining
Fix the neonatal diaphragm in a 12-well plate with 4% PFA for 1-2 h at room temperature (this step can be omitted as embryonic diaphragms were isolated from embryos fixed with 4% PFA overnight at 4°C).
Wash the diaphragm with PBS three times, 5-8 min each, at room temperature, and shaking slightly at 50-60 rpm.
Wash the diaphragm with 0.1 M glycine for 1 h at room temperature, shaking slightly at 50-60 rpm (a long time fixation will increase fixation-induced autofluorescence, and 0.1 M glycine can reduce the autofluorescence).
Wash the diaphragm with PBS three times, 5-8 min each, at room temperature, and shaking slightly at 50-60 rpm.
Permeabilize the diaphragm with 0.5% Triton X-100 in PBS for 1 h at room temperature without shaking. This step is especially critical for the following blocking and staining procedures.
Transfer the diaphragm onto the glass slide and draw a circle around the outside of the diaphragm with a hydrophobic pen. Then, block it with 100 μl blocking solution overnight at 4°C in a black incubator box containing a wet towel inside (Figure 1).
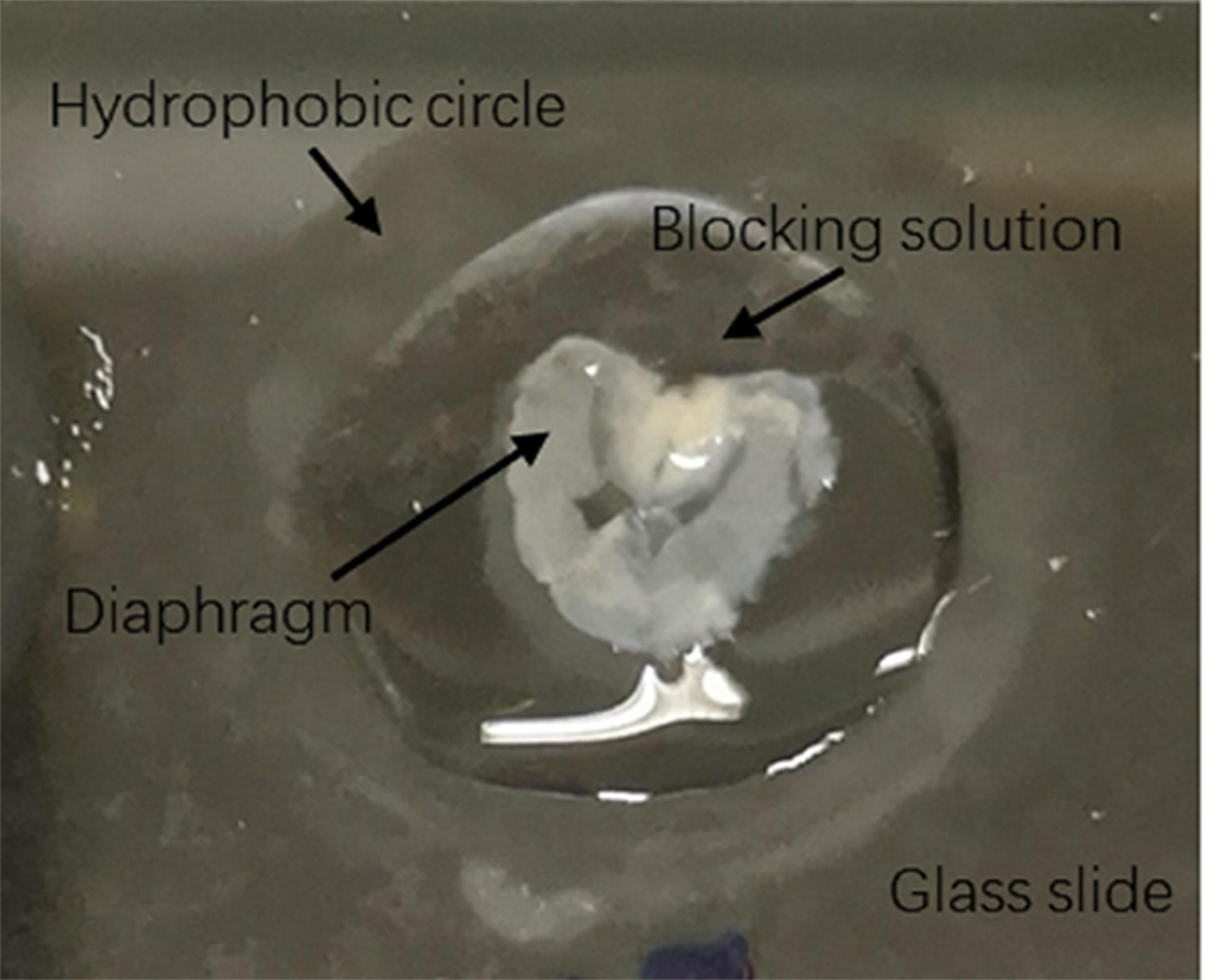
Figure 1. Method of embryonic/neonatal diaphragm blockingRemove the blocking solution and add the primary antibody solution. Incubate it overnight at 4°C in a black wet incubator box.
Remove the primary antibody solution and wash the diaphragm with 0.5% Triton X-100 in PBS three times, 1 h each, at room temperature.
Incubate overnight at 4°C with a-BTX and fluorochrome-conjugated secondary antibodies diluted in blocking solution.
Remove the secondary antibody solution and wash the diaphragm with 0.5% Triton X-100 in PBS three times, 1 h each, at room temperature.
Mount the diaphragm with 100 μl of mounting medium and cover it with a coverslip. Store it at 4°C overnight until confocal imaging.
Adult diaphragm staining
Make a small hole (4 × 4 × 2 cm) on the foam board covered with aluminum foil and place the diaphragm inside; pin it in place with needles and fix it with 4% PFA for 1-2 h at room temperature (Figure 2).
Note: This step should be performed immediately after the diaphragm separation.
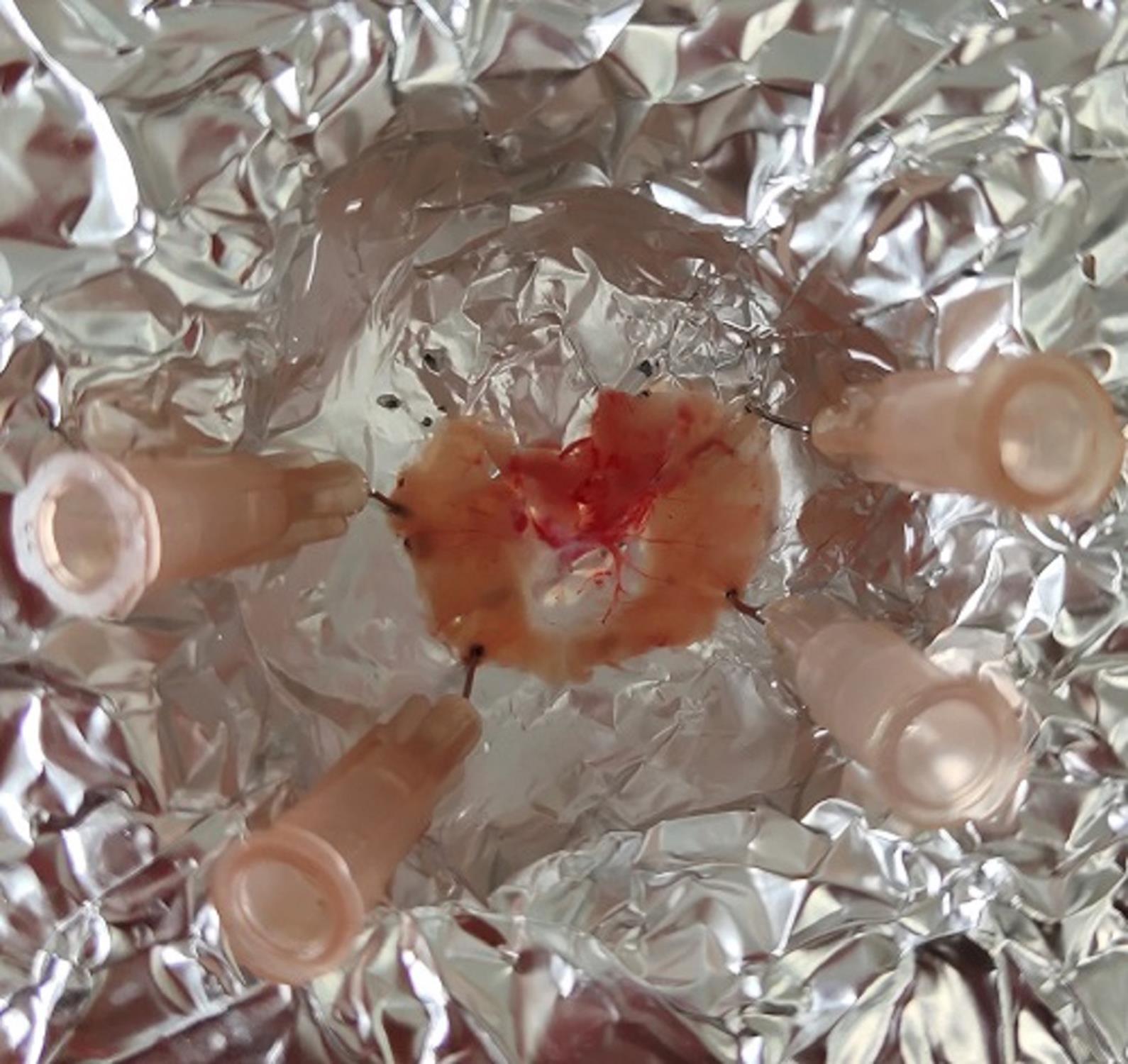
Figure 2. Method of adult diaphragm fixationWash the diaphragm with PBS three times, 5-8 min each, at room temperature, and shaking slightly at 50-60 rpm.
Wash the diaphragm with 0.1 M glycine for 1 h at room temperature, shaking slightly at 50-60 rpm. For adult diaphragms, carefully remove connective tissues that cover their surface with arrow forceps at this step (Figure 3).
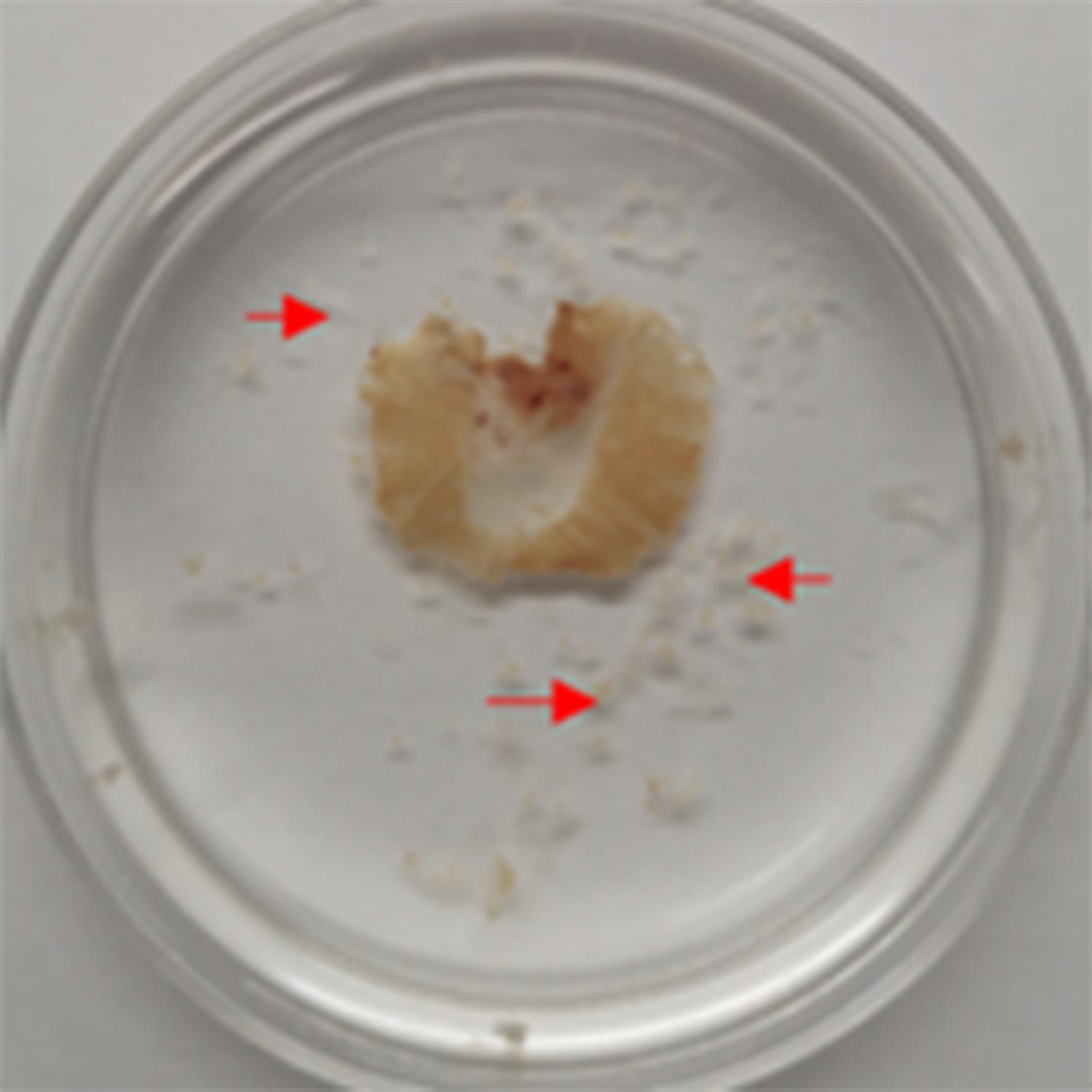
Figure 3. Removal of connective tissues from the adult diaphragm. Debris of removed connective tissues are indicated by red arrows.Wash the diaphragm with PBS three times, 5-8 min each, at room temperature, and shaking slightly at 50-60 rpm.
Permeabilize the diaphragm with 0.5% Triton X-100 in PBS overnight at 4°C without shaking.
Perform blocking in a 12-well plate with 500 μl blocking solution at room temperature without shaking (Figure 4).
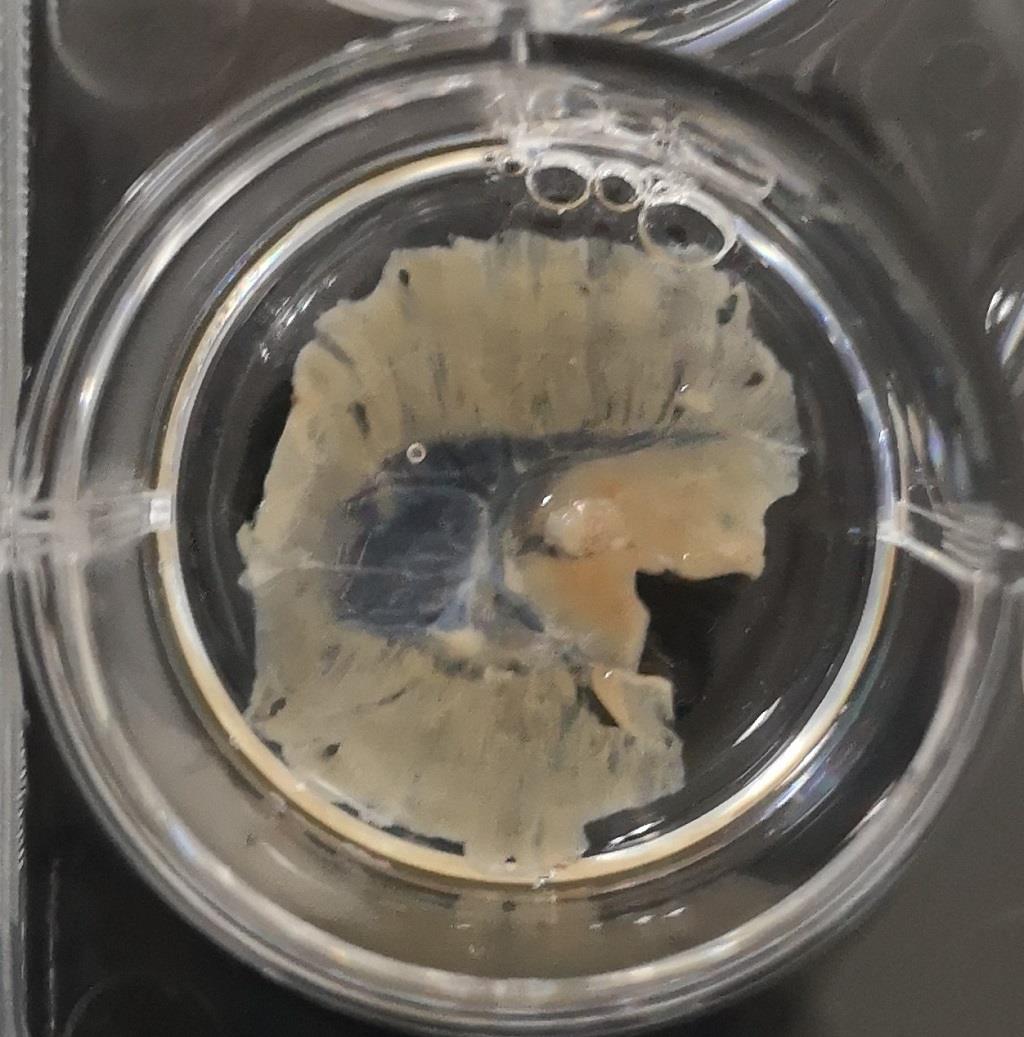
Figure 4. Method of adult diaphragm blockingThe remaining staining procedures are the same as for Steps B1g to B1k, Embryonic/neonatal staining. For an adult diaphragm, keep pipetting the antibody solution onto the middle of the diaphragm for 5 min to allow the antibody to penetrate into the diaphragm. Then, incubate overnight at 4°C.
Data analysis
Images of E16.5 diaphragm NMJ staining are shown in Figure 5. Presynaptic nerve terminals were labeled with anti-synaptophysin (SYN, red), and post-synapse AChRs were labeled with α-Bungarotoxin (α-BTX, green). Images were taken using a Zeiss Confocal Laser-scanning Microscope at 20x magnification. Note that majority of nerve terminals were colocalized with AChRs in the embryonic E16.5 diaphragm.
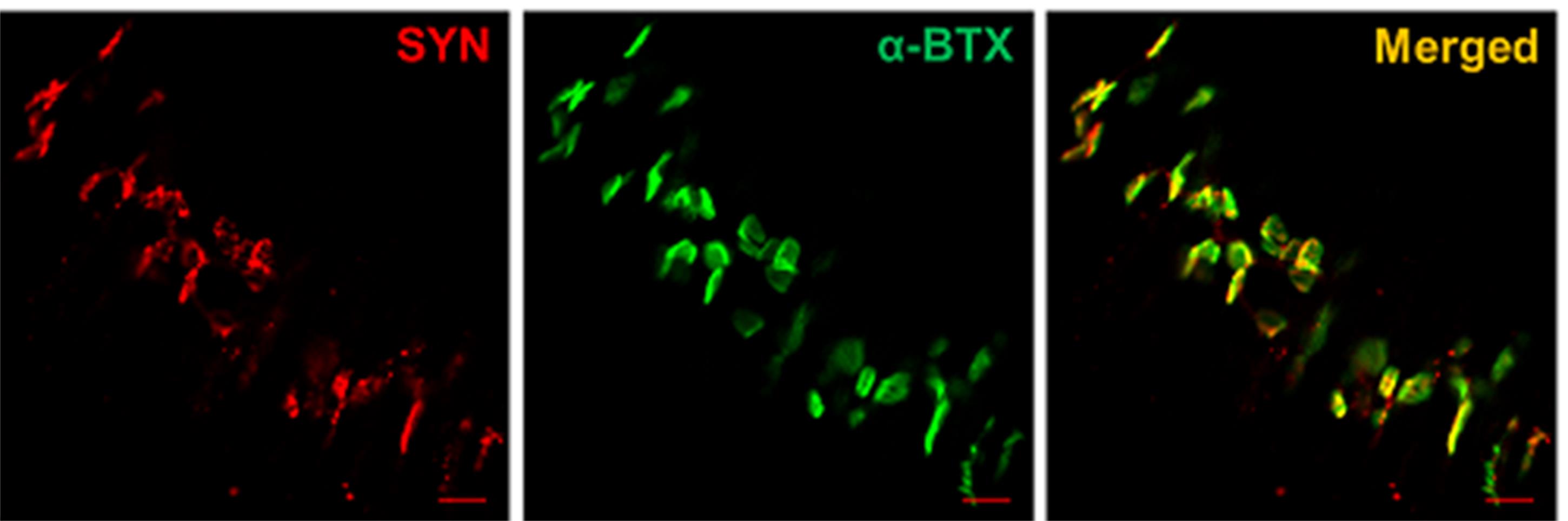
Figure 5. The NMJ immunofluorescence staining of E16.5 diaphragm. Scale bars: 20 μm.
Recipes
10× PBS
For 1 L solution, dilute 80 g of NaCl, 2 g of KCl, 35.8 g of Na2HPO4·12H2O, and 2.4 g of KH2PO4 in 1 L ddH2O. Shake vigorously at room temperature until completely dissolved and store at room temperature. Dilute ten times when using 1× PBS.
4% paraformaldehyde (PFA)
Dilute 0.4 g of paraformaldehyde in 10 ml of 1× PBS. Place it at 60°C for 1-2 h until completely dissolved and then put in 4°C before use. The 4% PFA solution should be prepared freshly.
0.5% Triton X-100
Combine 2.5 ml of 100% Triton X-100 with 500 ml of 1× PBS. Shake vigorously at room temperature until completely dissolved.
0.1 M glycine
Dilute 0.375 g of glycine in 50 ml of 1× PBS. Shake vigorously at room temperature until completely dissolved.
Blocking solution
5% normal goat serum (NGS). For 5 ml solution, dilute 250 μl of 100% NGS in 4.75 ml of 0.5% Triton X-100. Pipette and mix well.
Primary antibody solution
Dilute 1 μl anti-synaptophysin antibodies in 100 μl blocking solution.
Secondary antibody solution
Dilute α-Bungarotoxin-ATTO Fluor-488 and goat anti-rabbit IgG Alexa FluorTM 546 conjugate in the blocking solution. The final dilution of α-Bungarotoxin-ATTO Fluor-488 is 1:100 and goat anti-rabbit IgG Alexa FluorTM 546 conjugate is 1:1,000.
Acknowledgments
This work was supported by grants from the Shanghai Scientific Research Project (19JC1416000), the National Natural Science Foundation (31870819, 31570818). This protocol was adapted from Liu et al. (2020).
Competing interests
There are no conflicts of interest or competing interests.
References
- Arimura, S., Okada, T., Tezuka, T., Chiyo, T., Kasahara, Y., Yoshimura, T., Motomura, M., Yoshida, N., Beeson, D., Takeda, S. et al. (2014). Neuromuscular disease. DOK7 gene therapy benefits mouse models of diseases characterized by defects in the neuromuscular junction. Science 345(6203): 1505-1508.
- Li, L., Xiong, W. C. and Mei, L. (2018). Neuromuscular Junction Formation, Aging, and Disorders. Annu Rev Physiol 80159-188.
- Liu, W. and Chakkalakal, J. V. (2018). The Composition, Development, and Regeneration of Neuromuscular Junctions. Curr Top Dev Biol 12699-124.
- Liu, Y., Luo, Y., Shen, L., Guo, R., Zhan, Z., Yuan, N., Sha, R., Qian, W., Wang, Z. and Xie, Z. (2020). Splicing Factor SRSF1 Is Essential for Satellite Cell Proliferation and Postnatal Maturation of Neuromuscular Junctions in Mice. Stem Cell Reports 15(4): 941-954.
- Merrell, A. J. and Kardon, G. (2013). Development of the diaphragm-- a skeletal muscle essential for mammalian respiration. FEBS J 280(17): 4026-4035.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sha, R., Wang, Z., You, X., Liu, Y., Xie, Z. and Feng, Y. (2021). Whole-mount Staining of Mouse Diaphragm Neuromuscular Junctions. Bio-protocol 11(21): e4215. DOI: 10.21769/BioProtoc.4215.
Category
Neuroscience > Sensory and motor systems > Animal model
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









