- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
High Throughput Analyses of Ascorbate-turnover Enzyme Activities in Rice (Oryza sativa L.) Seedlings
Published: Vol 11, Iss 20, Oct 20, 2021 DOI: 10.21769/BioProtoc.4190 Views: 4328
Reviewed by: Agnieszka ZienkiewiczAyelign M. AdalLifeng Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Semi-throughput Procedure for Assaying Plant NADP-malate Dehydrogenase Activity Using a Plate Reader
Kevin Baudry and Emmanuelle Issakidis-Bourguet
Aug 20, 2023 1466 Views

An in vitro Assay to Probe the Formation of Biomolecular Condensates
Yu Zhang and Shen Lisha
Sep 5, 2023 3198 Views

Immunofluorescence for Detection of TOR Kinase Activity In Situ in Photosynthetic Organisms
Ana P. Lando [...] Giselle M. A. Martínez-Noël
Dec 20, 2024 1813 Views
Abstract
Ascorbate (Vitamin C) fulfills various functions in plant photosynthesis and abiotic stress tolerance. The four key enzymes involved in the ascorbate-turnover pathway are ascorbate peroxidase, ascorbate oxidase, monodehydroascorbate reductase, and dehydroascorbate reductase. Several reports have shown the pivotal roles of these enzymes in plant development and stress tolerance. Therefore, reliable and rapid assay protocols are required for researchers to investigate their enzymatic activities during plant development and stress responses. Previously published methods for analyzing these enzymatic activities rely on cuvette spectrophotometers, which can only handle one sample per test, leading to a prolonged investigation. In this protocol, we employed a 96-well microplate reader to analyze at least eight samples with two technical replicates simultaneously. We analyzed two rice (Oryza sativa L.) genotypes with distinct ascorbate oxidase and dehydroascorbate reductase activities to demonstrate the assay process, including plant growth, sample preparation, reaction setup, and data analysis. Our protocol provides a high throughput method for investigating ascorbate turnover-related enzymatic activities in plants.
Keywords: Ascorbic acidBackground
L-Ascorbic acid (AsA, vitamin C) is the most abundant water-soluble antioxidant involved in plant photosynthesis, hormone biosynthesis, and abiotic stress tolerance (Gallie, 2012). In photosynthesis, AsA functions as the redox state regulator of photosynthetic electron carriers through ascorbate peroxidase (APX, EC 1.11.1.11) (Smirnoff and Wheeler, 2000). Ascorbate oxidase (AO, EC 1.10.3.3) catalyzes the oxidation of AsA to monodehydroascorbic acid (MDHA), which further disproportionate into dehydroascorbic acid (DHA) and AsA. MDHA and DHA can be recycled into AsA by monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) and dehydroascorbate reductase (DHAR, EC 1.8.5.1), respectively (Figure 1). The turnover of AsA plays an essential role in abiotic stress tolerance in plants. Over-expressing an APX gene in sweet potato (Ipomoea batatas) conferred tolerance to salt, oxidative, and chilling stresses (Lim et al., 2007; Yan et al., 2016). Similarly, over-expressing an MDHAR gene in tomato plants increased the tolerance to chilling stress (Stevens et al., 2008). Moreover, Johnston et al. (2015) identified the roles of plant MDHAR in the mediation of 2,4,6-trinitrotoluene (TNT) toxicity. Wu et al. (2017) reported that iron toxicity tolerance in rice is associated with higher AO activity and lower DHAR activity. Due to the pivotal relevance in plant development and stress tolerance, reliable and rapid assay protocols are required for investigating the roles of these AsA-turnover enzymes in plants under various conditions. An AO activity assay in Tobacco (Nicotiana tabacum L.) was reported by Pignocchi et al. (2003) using a photospectrometric method to monitor the AsA oxidation rate indicated by the decrease of absorbance at 265 nm. For APX activity assay, Das et al. (2015) measured the H2O2-mediated oxidation of AsA by analyzing the absorbance at 290 nm using a spectrophotometer. MDHAR activity in spinach was determined by Hossain et al. (1984), in which they measured the oxidation of NADH through monitoring the decrease of absorbance at 340 nm. For DHAR activity assay, Stahl et al. (1983) reported a protocol using a spectrophotometric method to examine the generation of AsA. These protocols typically employed spectrophotometers and cuvettes to monitor absorbance change at a specific wavelength, only measuring one sample per run leading to the prolonged investigation. To overcome the limitations of low throughput in previously reported protocols, we developed a microplate reader-based method capable of measuring more than eight samples with technical replicates in one test. Our protocol can also be adapted for investigating ascorbate turnover-related enzymes in other plant species.
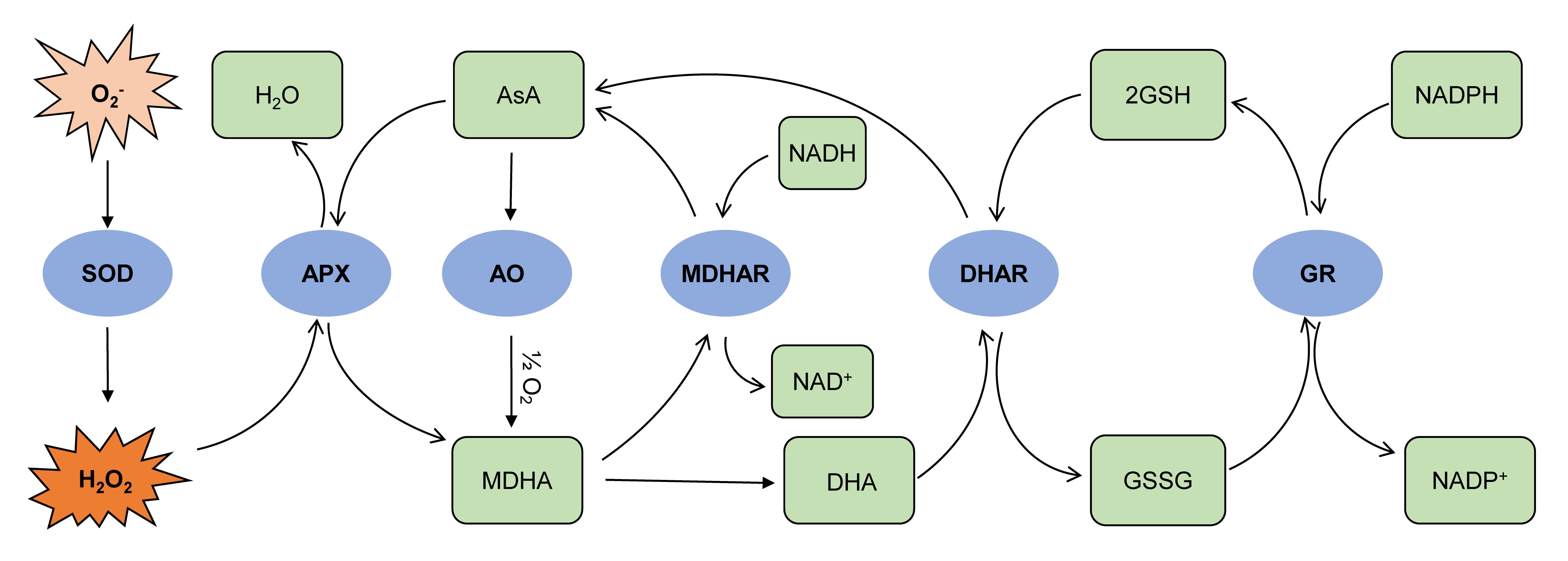
Figure 1. Ascorbate-turnover pathway and ascorbate-glutathione cycle. In the chloroplast, SOD scavenges O2- produced in PSII to form O2 and H2O2 molecules. APX mediates the scavenging of H2O2 with AsA as the electron donor, leading to the generation of MDHA. MDHA is also generated through the oxidation of AsA mediated by AO. MDHA further involves two distinct processes: (i) being reduced back to AsA through MDHAR with NADH as the electron donor, and (ii) being dissociated to generate DHA. DHAR mediates the reduction of DHA to AsA with two molecules of GSH as the electron donor. GR regulates the reduction of GSSG to GSH with NADPH as the electron donor. O2-, superoxide; SOD, superoxide dismutase; H2O2, hydrogen peroxide; APX, ascorbate peroxidase; AsA, ascorbic acid/ascorbate; AO, ascorbate oxidase; MDHA, monodehydroascorbate; MDHAR, monodehydroascorbate reductase; NADH/NAD+, nicotinamide adenine dinucleotide; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; GSH, glutathione; GSSG, glutathione disulfide; GR, glutathione reductase; NADPH/NADP+, nicotinamide adenine dinucleotide phosphate.
Materials and Reagents
Plant materials
Rice seedlings of IR29 and FL483 used in this protocol were described in our previous publication (Wu et al., 2017). FL483 is a recombinant inbred line derived from the cross between IR29 (Oryza sativa L., ssp. indica) and Pokkali (ssp. indica). IR29 and FL483 showed contrasting AO and DHAR activities, further presented in the Data analysis section.
Chemicals and reagents
L-(+)-Ascorbic acid, C6H8O6 (Alfa Aesar, catalog number: A15613), store at 2-8°C
Ascorbate oxidase, Cucurbita sp. (Merck, catalog number: 189724), store at -20 °C
Bovine serum albumin, BSA (Sigma-Aldrich, catalog number: 05470); store at 2-8°C
Bradford reagent (Sigma-Aldrich, catalog number: B6916); store at 2-8°C
Dehydro-L-(+)-ascorbic acid dimer, C12H12O12 (Sigma-Aldrich, catalog number: D8132), store at -20°C
EDTA disodium salt 2-hydrate, C10H14N2Na2O8·2H2O (PanReac AppliChem, catalog number: A2937), store at room temperature (RT)
L-Glutathione reduced, C10H17N3O6S (Sigma-Aldrich, catalog number: G4251), store at 2-8°C
Glycerol (Merck, catalog number: G5516-100ML), store at RT
Hydrogen chloride, HCl (Carl Roth, catalog number: 7476.1), store at RT
Hydrogen peroxide, H2O2 (Merck, catalog number: 386790-100ML), store at 2-8°C in the dark
Magnesium chloride hexahydrate, MgCl2·6H2O (Sigma-Aldrich, catalog number: M9272), store at 2-8°C
β-Nicotinamide adenine dinucleotide, reduced disodium salt hydrate, NADH (Sigma-Aldrich, catalog number: N8129), store at -20°C
Potassium phosphate dibasic, K2HPO4 (Sigma-Aldrich, catalog number: P3786), store at RT
Potassium phosphate monobasic, KH2PO4 (Sigma-Aldrich, catalog number: P0662), store at RT
Sodium chloride, NaCl (Sigma-Aldrich, catalog number: 31434), store at RT
Sodium phosphate monobasic monohydrate, NaH2PO4·H2O (Sigma-Aldrich, catalog number: S9638), store at RT
Sodium phosphate dibasic dehydrate, Na2HPO4 (Sigma-Aldrich, catalog number: 71643), store at RT
Tris ultrapure, C4H11NO3 (PanReac AppliChem, catalog number: A1086), store at RT
Fertilizer for rice plants (ICL/SF, Peter Excel CalMag Grower, catalog number: 2152), store at RT
Artificial soil for rice growth (see Table 2 in Recipes)
AO extraction buffer for soluble fraction (see Recipes), store at RT
AO extraction buffer for ionically bound fraction (see Recipes), store at RT
AO assay buffer (see Recipes), store at RT
APX/MDHAR extraction buffer (see Recipes), prepare fresh
APX assay buffer (see Recipes), prepare fresh
AO from Cucurbita sp. enzyme solution (see Recipes), store at -20°C
MDHAR assay buffer (see Recipes), prepare fresh
DHAR extraction buffer (see Recipes), store at RT
DHAR assay buffer (see Recipes), store at RT
Consumables
Pipette tips 0.1-10 µl (Eppendorf, epT.I.P.S.® Standard, catalog number: 0030000811)
Pipette tips 2-200 µl (Eppendorf, epT.I.P.S.® Standard, catalog number: 0030000889)
Pipette tips 100-1,000 µl (Eppendorf, epT.I.P.S.® Standard, catalog number: 0030000927)
Centrifuge tubes 1.5 ml (Eppendorf, Safe-Lock Tubes, catalog number: 0030120086)
Centrifuge tubes 2.0 ml (Eppendorf, Safe-Lock Tubes, catalog number: 0030120094)
Centrifuge tubes 50 ml (Corning, Falcon® Tubes, catalog number: 352070)
Filter paper (Schleicher and Schuell, catalog number: 311609)
UV-transparent 96-well microplate (Greiner Bio-One, UV-Star® Microplate, catalog number: 655801)
Microplate (Greiner Bio-One, 96 well Microplate, catalog number: 655101)
Serological pipet 5 ml (Eppendorf, Serological Pipets, catalog number: 0030127714)
Serological pipet 25 ml (Eppendorf, Serological Pipets, catalog number: 0030127730)
Serological pipet 50 ml (Eppendorf, Serological Pipets, catalog number: 0030127749)
Parafilm (Bemis, Parafilm M, catalog number: 2910057)
PCR strips (Biozym, PCR SingleCap 8er-SoftStrips 0.2 ml, catalog number: 710970)
Petri dishes (Greiner Bio-One, 100 × 20 mm, catalog number: 664102)
Reservoir (Corning, Axygen® 25 ml disposable, catalog number: RES-V-25)
Plant growth pots, 2 L (Nitsch, round pots, catalog number: 503402)
Holding trays for plant growth (Hermann Meyer, catalog number: 749651)
Equipment
Note: Other equipment models with similar functions, for example, microplate reader with absorbance measurement capabilities covering the spectral range of 265-595 nm, centrifuge with cooling function, multichannel pipettes, can also be used instead of the models listed. In this protocol, we described the assays of rice shoot samples, which require pestle and mortar for grinding. For smaller amounts of tissues, researchers can also use 2 ml centrifuge tubes and a tissue grinder for sample preparation.
-80°C freezer (Thermo Scientific, TSX ULT freezer, catalog number: TSX70086V)
Centrifuge with cooling function (Eppendorf, Centrifuge 5425R, catalog number: 5406000216)
Lab incubator (Memmert, Universal oven, catalog number: UN110m)
Analytical lab balance (Sartorius, 1702 analytical balance, catalog number: 05771)
Benchtop pH meter (Xylem Analytics, WTWTM inolab® 7110, catalog number: 9920090)
Ice bucket
Lab vortex mixer (Scientific Industries, Vortex-Genie 2, catalog number: SI-0236)
Magnetic stirrer (IKA, RH basic 2, catalog number: 0003339000)
Pipette controller (Eppendorf, Easypet 3, catalog number: 4430000018)
Multichannel pipette 0.5-10 µl (Eppendorf, Research®plus, catalog number: 3125000010)
Multichannel pipette 10-100 µl (Eppendorf, Research®plus, catalog number: 3125000036)
Pipette 2-20 µl (Eppendorf, Research®plus, catalog number: 3123000039)
Pipette 100-1,000 µl (Eppendorf, Research®plus, catalog number: 3123000063)
Microplate reader (TECAN; Infinite® 200 Pro M Plex, catalog number: INF-MPLEX)
Volumetric flasks 100 ml (Merck, Duran®, catalog number: DWK213722473)
Volumetric flasks 250 ml (Merck, Duran®, catalog number: DWK213723675)
Volumetric flasks 500 ml (Merck, Duran®, catalog number: DWK213724474)
Laboratory bottles 100 ml with screw cap and pouring ring (Merck, Duran®, catalog number: Z305170)
Laboratory bottles 250 ml with screw cap and pouring ring (Merck, Duran®, catalog number: Z305189)
Laboratory bottles 500 ml with screw cap and pouring ring (Merck, Duran®, catalog number: Z305197)
Pestle and mortar (Fisher Scientific, Haldenwanger 55-2, catalog number: 15292519)
Climate-controlled glasshouse
Software
Microplate reader i-controlTM (TECAN, https://lifesciences.tecan.com)
Microsoft Excel (Microsoft, https://www.microsoft.com/en-ww/microsoft-365/excel)
Procedure
Figure 2 briefly demonstrates the whole procedure described in this protocol. In addition, Figure 3 shows a workflow describing detailed steps for analyzing different ascorbate turnover enzyme activities.
Grow rice plants in the climate-controlled glasshouse
Place 15-20 seeds on the filter paper soaked with deionized water in one Petri dish; seal the Petri dish with parafilm and germinate in the dark at 30°C for three days.
Fill the 2 L pots with rice growth soil and soak the soil thoroughly with tap water.
Carefully transplant the 3-day-old rice seedlings into 2 L pots, each pot with one seedling.
Note: Be gentle not to damage the seedlings and cover the roots with 1-2 cm soil.
Set up the rice growth conditions as following: day/night temperature, 28/22°C; relative humidity, 60-70%; light intensity, 500-600 µmol m-2 s-1; and day/night period, 8/16 h (short-day).
Check the growth pots regularly to ensure sufficient standing water; apply 20 ml of 15 g/L Peter Excel fertilizer solution weekly to each tray from when the seedlings are 2 weeks old.
Harvest and flash-freeze the aboveground shoots of 3-week-old plants with liquid nitrogen.
Grind around 1-2 g of harvested samples to a fine powder using pre-chilled pestle and mortar; store them in the sterile 15 ml Falcon tubes and place them in the -80°C freezer upon extraction.
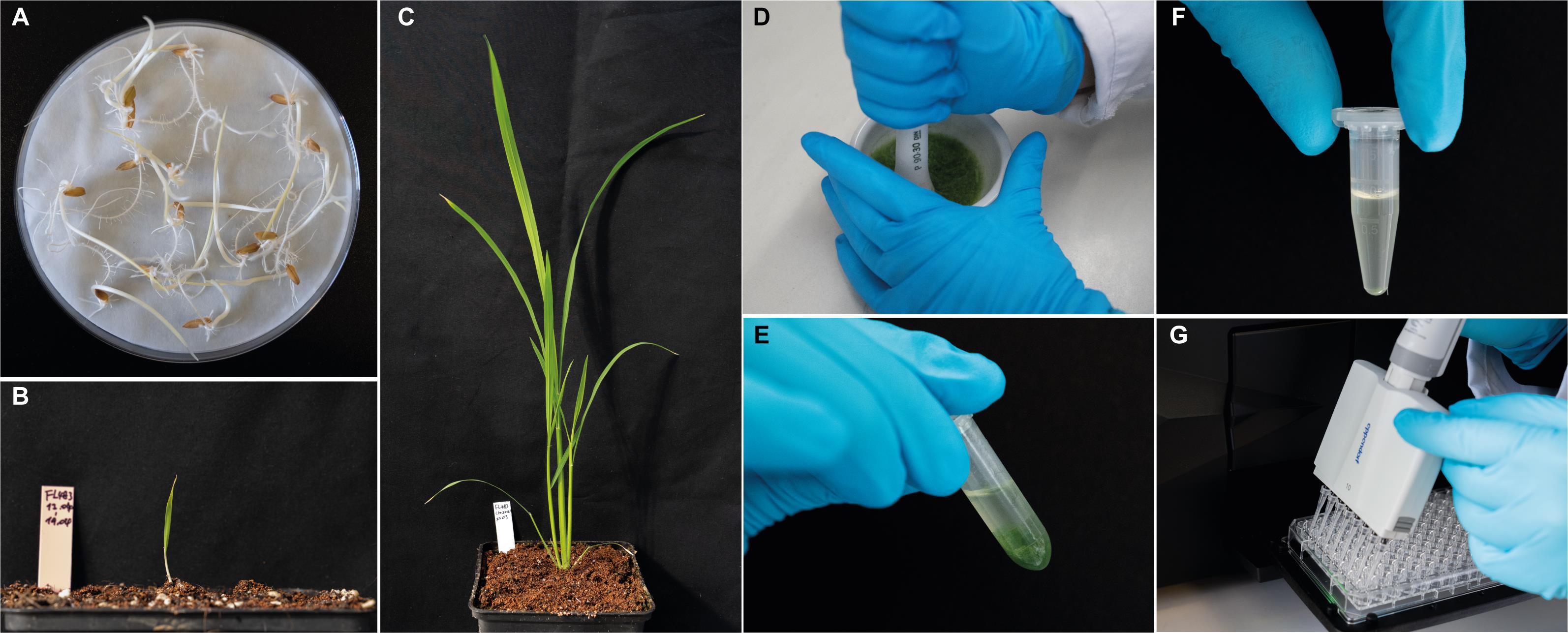
Figure 2. Representative photos summarizing the working procedure described in this protocol. (A) Germinate 15-20 seeds on soaked filter paper at 30°C in the dark for 3 days. (B) Transplant 3-day-old seedlings into pots filled with rice growth soil. (C) Grow the plants for 3 weeks and harvest the shoots with liquid nitrogen. (D) Grind the plant sample in liquid nitrogen with a pestle and mortar. (E) Weigh 80-100 mg of sample into centrifuge tubes and add extraction buffer. (F) Transfer the supernatant into a new tube after centrifugation at 4°C. (G) Set up the enzymatic reaction by mixing assay buffer, plant extracts, and reaction substrate; monitor the substrate/product change through absorbance kinetics measurement.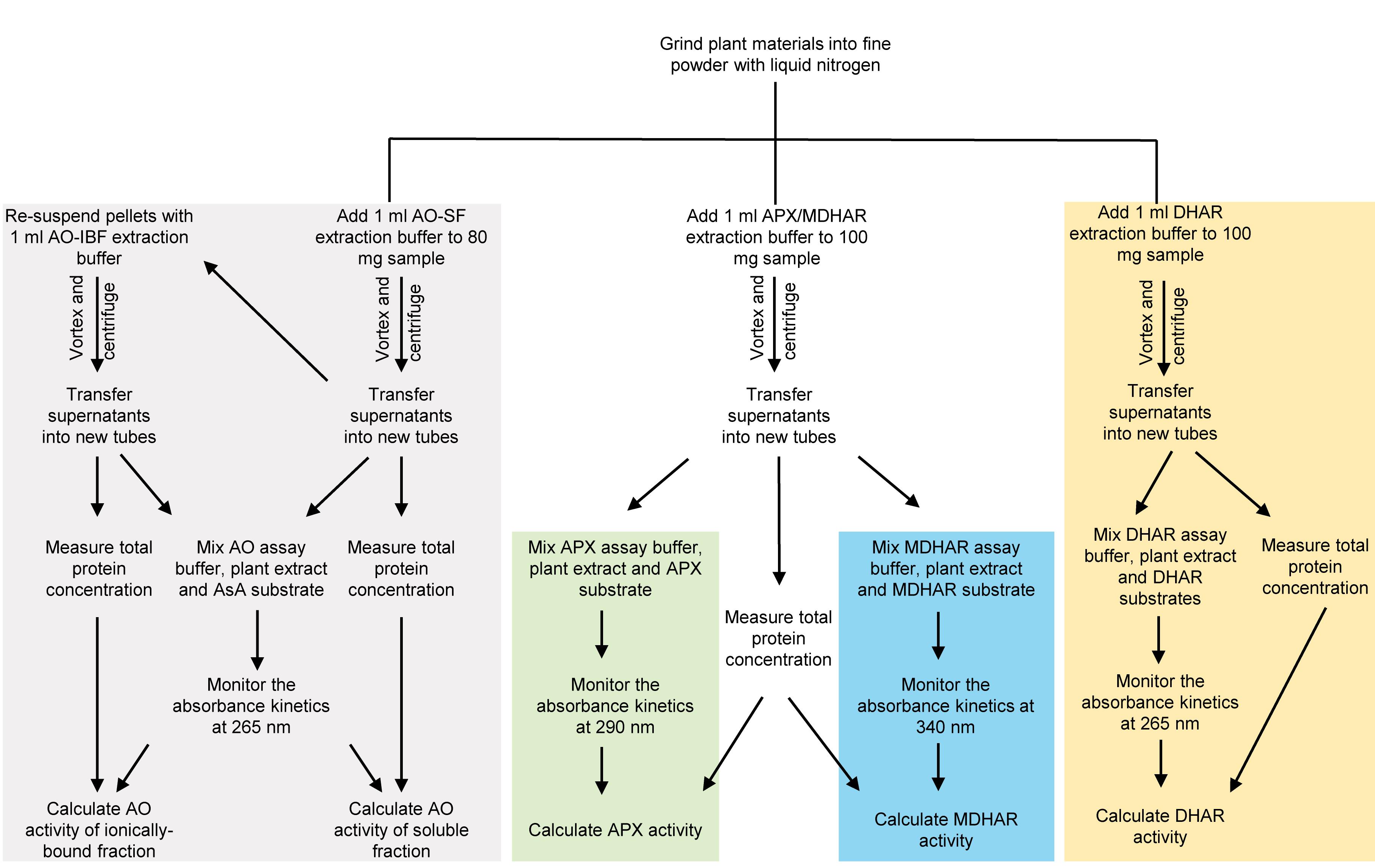
Figure 3. Summary of the assay steps for ascorbate-turnover enzyme activities described in this protocol. Grind plant material into a fine powder with liquid nitrogen for further assays. Apply extraction buffer to prepare plant extracts for target enzymatic assay. The gray background highlights the steps for both AO-SF and AO-IBF activities. APX (highlighted with green background) and MDHAR (highlighted with blue background) assays share the same extraction steps, while the assay steps are different. The yellow background highlights the steps for DHAR assay. Further details of assays and enzymatic calculations are described in the following sections (see Procedures B-D, Data analysis, and Recipes).AO-SF, ascorbate oxidase soluble fraction; AO-IBF, ascorbate oxidase ionically bound fraction; APX, ascorbate peroxidase; MDHAR, monodehydroascorbate reductase; DHAR, dehydroascorbate reductase.
AO activity assay for the soluble and ionically bound fraction
Weigh 80 mg of ground samples in pre-chilled 2 ml centrifuge tubes; add 1 ml of AO extraction buffer for soluble fraction (AO-SF).
Vortex the mixture vigorously for 30 s and place the tubes on ice.
Centrifuge at 14,000 × g for 10 min at 4°C.
Transfer the supernatant into new 1.5 ml tubes for AO soluble fraction activity assay; centrifuge the supernatant again for 1 min to clear the extracts.
Note: We recommend analyzing the samples immediately after extraction. However, extracted samples can be stored in -20°C freezer for up to one week or in -80°C freezer for up to one month before analysis. Steps B5-B6 are for AO ionically bound fraction extraction; assay steps are identical for both soluble and ionically bound fractions of AO.
Resuspend the pellets with 1 ml of AO extraction buffer for ionically bound fraction (AO-IBF); vortex vigorously for 20 s.
Centrifuge the extracts at 14,000 × g for 10 min at 4°C and transfer the supernatant into new 1.5 ml tubes placed on ice.
Mix 80 µl of AO assay buffer and 10 µl of plant extract in the UV-transparent 96-well microplate; prepare two analytical replicates for each sample.
Start the reaction by adding 10 µl of 2 mM AsA using a multichannel pipette; mix well by pipetting up and down 6-8 times.
Monitor the kinetics of absorbance at 265 nm for 5 min at 25°C.
Prepare a series of BSA standard solutions using AO extraction buffer: 0, 0.1, 0.2, 0.5, and 1.0 mg/ml.
Note: To determine protein concentration in AO soluble fraction extracts, use AO-SF extraction buffer (without 1 M NaCl, see Recipes) to dissolve BSA; for AO ionically bound fraction extracts, use AO-IBF extraction buffer (with 1 M NaCl, see Recipes) to dissolve BSA.
Mix 25 µl of BSA standards or plant extracts with 475 µl Bradford reagent solutions; vortex for 15 s and incubate the mixture for 10 min at RT.
Load 100 µl of the reaction mixture into a 96-well microplate (non-UV transparent) and measure the absorbance at 595 nm wavelength; prepare two analytical replicates for both standards and plant extracts.
APX and MDHAR activity assay
Note: Use the same extraction buffer to prepare plant extracts for both APX and MDHAR assay.
Weigh 100 mg of sample into pre-chilled 2.0 ml tube; add 1 ml of APX/MDHAR extraction buffer.
Vortex vigorously for 30 s and centrifuge at 10,000 × g for 30 min at 4°C.
Transfer the supernatants into 1.5 ml tubes placed on ice; centrifuge for an additional 1 min to clear the extracts.
Note: We recommend analyzing the samples immediately after extraction. For APX and MDHAR activities assay, plant extracts are not suitable for a long period of storage in freezing conditions, which will decrease the enzymatic activities.
For the APX assay, thoroughly mix 80 µl of APX assay buffer and 10 µl of plant extracts in the UV-transparent microplate by pipetting up and down 6-8 times; prepare two analytical replicates for each sample.
Start the reaction by adding 10 µl of 0.03% (V/V) H2O2 solution; monitor the absorbance kinetics at 290 nm for 1 min at 25°C.
Note: The reaction is very rapid. Place the microplate in the reader, ready for immediate absorbance detection.
For the MDHAR assay, mix 80 µl of MDHAR assay buffer and 10 µl of plant extracts in the UV-transparent plate by pipetting up and down 6-8 times.
Start the reaction by adding 10 µl of 1 mM NADH; monitor the absorbance kinetics at 340 nm for 2 min at 25°C.
Note: The total protein concentration in the extracts for both APX and MDHAR assay are the same.
Prepare a series of BSA standard solutions with APX/MDHAR extraction buffer: 0, 0.1, 0.2, 0.5, and 1 mg/ml.
Mix 25 µl of BSA standard solution or plant extract with 475 µl of Bradford reagent in a 1.5 ml tube; vortex for 15 s and incubate at RT for 10 min.
Load 100 µl of the protein assay mixture into a 96-well microplate (non-UV transparent) and measure the absorbance at 595 nm; prepare two analytical replicates for each standard or sample.
DHAR activity assay
Weigh 100 mg of sample in pre-chilled 2 ml tubes; add 1 ml of DHAR extraction buffer.
Vortex vigorously for 30 s and centrifuge at 13,000 × g for 10 min at 4°C.
Transfer the supernatants into new 1.5 ml tubes placed on ice; re-centrifuge for 1 min to clear the extracts.
Note: We recommend analyzing the samples immediately after extraction. However, plant extracts can be stored in -20°C freezer for up to one week or -80°C freezer for up to one month before analysis.
Mix 70 µl of DHAR assay buffer, 10 µl of plant extracts, and 10 µl of 5 mM DHA solution into UV-transparent microplate by pipetting up and down 6-8 times; use DHAR extraction buffer for blanks; prepare two analytical replicates for each sample.
Note: It is essential to include the blanks in the DHAR assay since GSH can reduce DHA in the absence of DHAR.
Start the reaction by adding 10 µl of 50 mM GSH solution to the mixture; monitor the absorbance kinetics at 265 nm for 5 min at 25°C.
Prepare a series of BSA standards using DHAR extraction buffer: 0, 0.1, 0.2, 0.5, and 1.0 mg/ml.
Mix 25 µl of BSA standards or plant extracts with 475 µl Bradford reagent by vortexing for 15 s; incubate the mixture at RT for 10 min.
Load 100 µl of the mixture into the microplate (non-UV transparent) and measure the absorbance at 595 nm; prepare two analytical replicates for each standard and sample.
Data analysis
Enzymatic activity calculation relies on the amount of converted substrate or generated product per minute per mg of total protein (mol/min/mg protein). The absorbance kinetics of the reaction mixture reflects the substrate conversion or product generation rate (Figure 4). Use Beer’s law to calculate substrate/product concentration change rate within one minute in the reaction mixture.
Beer’s law: A = ɛlc, where
A is the absorbance;
ɛ is the molar extinction coefficient of substrate/product at a specific wavelength;
l is the optical path length in cm;
c is the concentration of substrate/product.
AO activity calculation for the soluble and ionically bound fraction
Determine the kinetic slopes of absorbance at 265 nm; check the linear correlation coefficient (Figure 4A).
Note: The coefficient of determination R2 should be greater than 0.99 for a reliable AO activity assay.
Apply Beer’s law to calculate the rate of AsA oxidation per minute. Divide the absolute values of slopes (A, OD min-1) by ɛ265 nm (14 mM-1 cm-1) and optical path length l (0.278 cm in this protocol) to obtain the concentration change of AsA within one minute.
Note: For other types of 96-well microplate, calculate the optical path length for 100 µl solution referring to the product’s datasheet.
Multiply the change of AsA concentration per minute with the volume (100 µl or 0.0001 L) of the reaction mixture to calculate the rate of AsA oxidation per minute.
Calculate the amount of total protein in 10 µl of plant extracts based on the standard curve (Figure 5).
Note: Before applying the linear correlation formula for protein concentration calculation, blank the sample absorbance value with 0 mg/ml standard absorbance value.
Calculate the AO activity using the rate of AsA oxidation per minute (Step A3) divided by the amount of protein in 10 µl of plant extracts (Step A4).
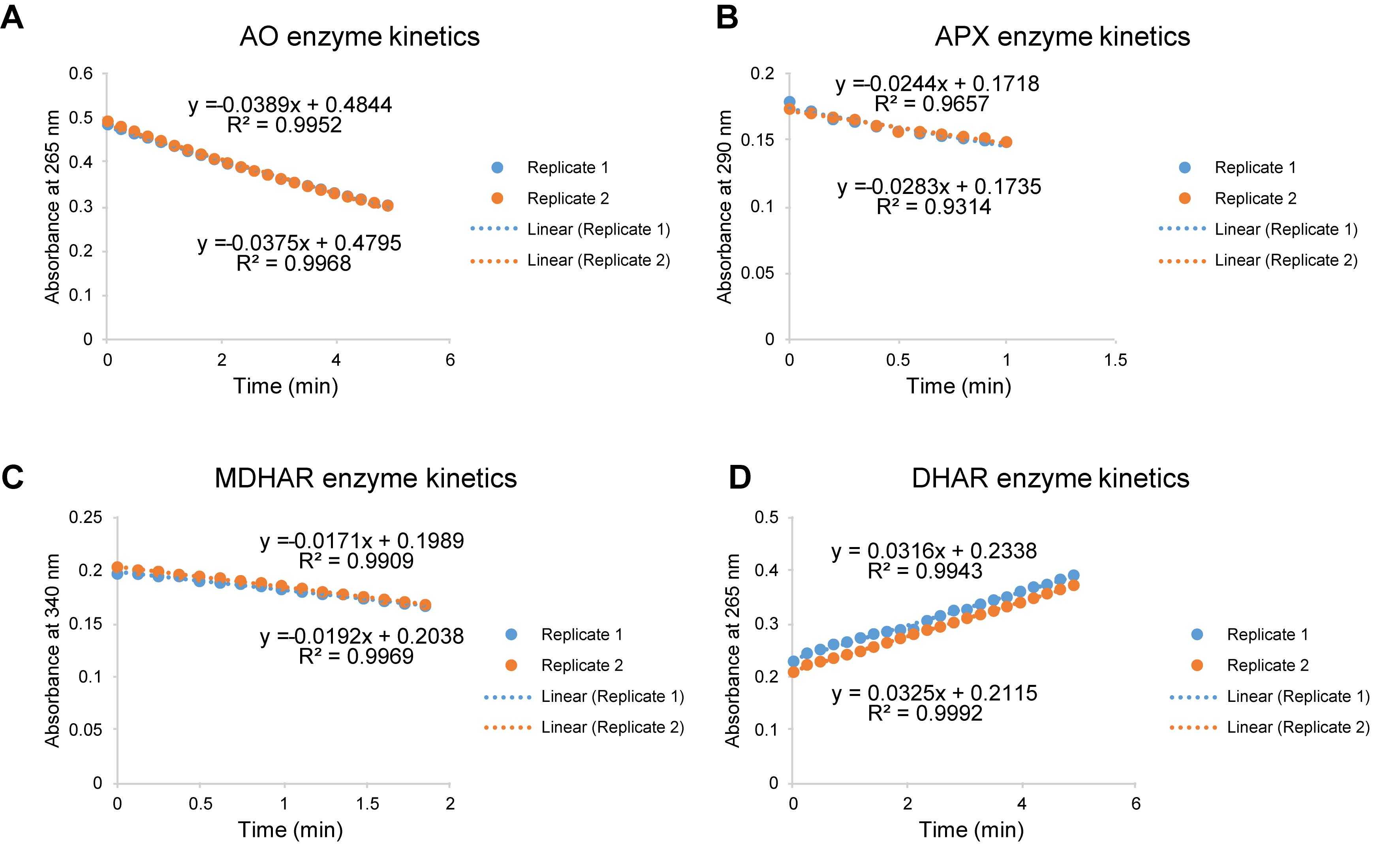
Figure 4. Scatter plots of absorbance-based kinetics for enzymatic activity assay. (A) AO assay by monitoring the absorbance kinetics at 265 nm to indicate the ascorbate oxidation rate. (B) APX assay by analyzing the absorbance kinetics at 290 nm. (C) MDHAR assay by measuring the absorbance kinetics at 340 nm to indicate NADH oxidation rate. (D) DHAR assay by measuring the absorbance kinetics at 265 nm to reveal ascorbate generation rate. Analyze linear correlation coefficient with Excel. Use the absolute values of kinetic slopes for enzymatic activity calculation. Two analytical replicates of each sample were shown. AO, ascorbate oxidase; APX, ascorbate peroxidase; MDHAR, monodehydroascorbate reductase; DHAR, dehydroascorbate reductase.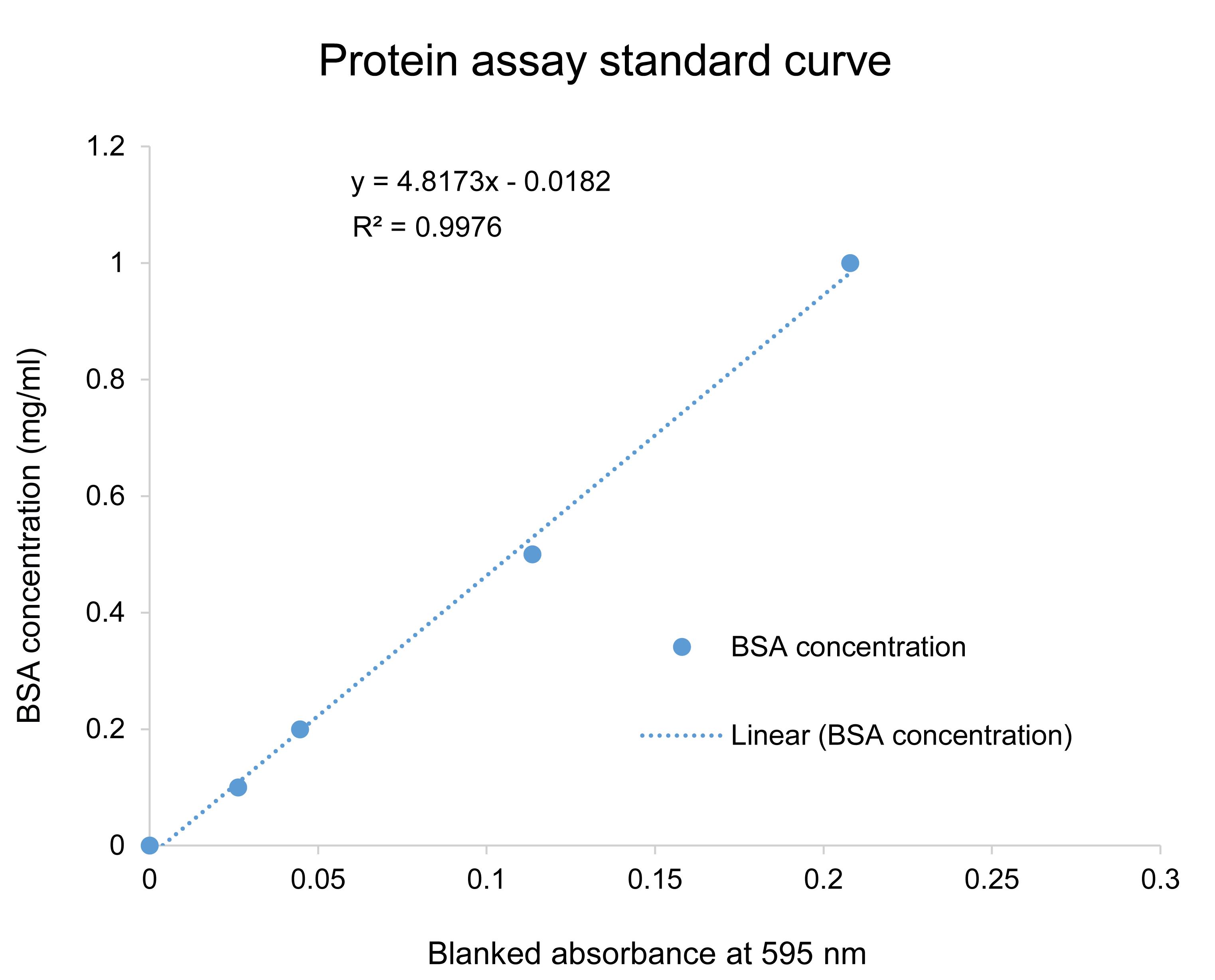
Figure 5. Standard curve for protein measurement in plant extracts. Generate the standard curve using BSA solutions with a series of concentrations ranging from 0.1 to 1.0 mg/ml prepared in an extraction buffer. The blank (0 mg/ml BSA) used in this curve is the extraction buffer for a specific enzymatic assay. Mix 25 µl of standard solution with 475 µl of Bradford reagent and incubate at RT for 10 min. Measure the absorbance at 595 nm with a microplate reader. Analyze the linear correlation between the absorbance values (blanked with 0 mg/ml BSA sample) with BSA concentrations.
APX activity calculation
Determine the kinetic slopes of absorbance at 290 nm; check the linear correlation coefficient (Figure 4B).
Note: The coefficient of determination R2 should be greater than 0.9 to obtain reliable APX activity results.
Apply Beer’s law to calculate the rate of AsA oxidation per minute. Divide the absolute values of slopes (A, OD min-1) by ɛ290 nm (2.8 mM-1 cm-1) and optical path length l (0.278 cm in this protocol) to obtain the concentration change of AsA within one minute.
Note: When using other types of 96-well UV-transparent microplate, calculate the optical path length for 100 µl of solution referring to the product's description.
Multiply the change of AsA concentration per minute with the reaction mixture volume (100 µl or 0.0001 L) to calculate the rate of AsA oxidation per minute.
Calculate the amount of total protein in 10 µl of plant extracts based on the standard curve prepared with BSA (Figure 5).
Note: Before applying the linear correlation formula for protein concentration calculation, blank the sample absorbance value with 0 mg/ml standard absorbance value.
Calculate the APX activity using the rate of AsA oxidation per minute (Step B3) divided by the amount of protein in 10 µl plant extracts (Step B4).
MDHAR activity calculation
Determine the slopes of absorbance kinetics at 340 nm; check the linear correlation coefficient (Figure 4C).
Note: The coefficient of determination R2 should be greater than 0.99 to obtain reliable MDHAR activity results.
Apply Beer’s law to calculate the rate of NADH oxidation per minute. Divide the absolute values of slopes (A, OD min-1) by ɛ340 nm (6.2 mM-1 cm-1) and optical path length l (0.278 cm in this protocol) to obtain the concentration change of NADH within one minute.
Note: When using other types of 96-well UV-transparent microplate, calculate the optical path length for 100 µl solution referring to the product’s description.
Multiply the change of NADH concentration per minute with the reaction mixture volume (100 µl or 0.0001 L) to calculate the rate of NADH oxidation per minute.
Calculate the amount of total protein in 10 µl of plant extracts based on the standard curve prepared with BSA (Figure 5).
Note: Before applying the linear correlation formula for protein concentration calculation, blank the sample absorbance value with 0 mg/ml standard absorbance value.
Calculate the MDHAR activity using the rate of NADH oxidation per minute (Step C3) divided by the amount of protein in 10 µl of plant extracts (Step C4).
DHAR activity calculation
Determine the kinetic slopes of absorbance at 265 nm in both samples and blanks; check the linear correlation coefficient (Figure 4D).
Note: The coefficient of determination R2 should be greater than 0.99 for a reliable DHAR activity assay. For the DHAR assay, it is essential to monitor the reaction in the blanks since GSH can reduce DHA in the absence of DHAR.
Subtract the slopes of samples with blanks; apply Beer’s law to calculate the rate of AsA generation per minute. Divide the absolute values of slopes (A, OD min-1) by ɛ265 nm (14 mM-1 cm-1) and optical path length l (0.278 cm in this protocol) to obtain the concentration change of AsA within one minute.
Note: For other types of 96-well microplate, calculate the optical path length for 100 µl solution refer to product’s datasheet.
Multiply the change of AsA concentration per minute with the reaction mixture volume (100 µl or 0.0001 L) to calculate the rate of AsA generation per minute.
Calculate the amount of total protein in 10 µl of plant extracts based on the standard curve prepared with BSA (Figure 5).
Note: Before applying the linear correlation formula for protein concentration calculation, blank the sample absorbance value with 0 mg/ml standard absorbance value.
Calculate the AO activity using the rate of AsA oxidation per minute (Step D3) divided by the amount of protein in 10 µl plant extracts (Step D4).
In the following Table 1, we describe the essential steps for calculating enzymatic activities using the absorbance kinetics slopes and protein concentration.
Table 1. Detailed steps for different enzymatic activities calculation
Target enzyme Absorbance kinetics slope
(OD min-1)Reaction rate in 100 µl mixture
(mmol min-1)Protein concentrate-on (mg/ml) Protein in 10 µl extract (mg) Enzymatic activity
(mmol min-1 mg-1)AO SAO RAO=SAO/ɛ265nm/l ×(100×10-6) PAO P’AO=PAO×(10×
10-3)EAO = RAO/P’AO APX SAPX RApx=SApx/ɛ290nm/l ×(100×10-6) PApx P’Apx=PApx×(10×10-3) EApx = RApx/P’Apx MDHAR SMDHAR RMDHAR=SMDHAR/ɛ340nm/l×(100×10-6) PMDHAR P’MDHAR=PMDHAR× (10×10-3) EMDHAR= RMDHAR/P’MDHAR DHAR SDHAR RDHAR=SDHAR/ɛ265nm/l ×(100×10-6) PDHAR P’DHAR=PDHAR× (10×10-3) EDHAR= RDHAR/P’DHAR Use Beer’s law to transform the absorbance kinetics slopes (Figure 4) into the substrate/product change rate. Determine the protein content in the 10 µl plant extract for enzymatic activity calculation. AO, ascorbate oxidase; APX, ascorbate peroxidase; MDHAR, monodehydroascorbate reductase; DHAR, dehydroascorbate reductase; ɛ265nm = 14 mM-1 cm-1; ɛ290nm = 2.8 mM-1 cm-1; ɛ340nm = 6.2 mM-1 cm-1; ɛ265nm = 14 mM-1 cm-1; l, optical path length; P, total protein concentration; P’, the amount of total protein in 10 µl extract; R, substrate/product conversion rate; E, enzymatic activity.
Statistical analysis and data presentation
In this protocol, we investigated one single factor: the genotype effect on enzyme activities. Employ One-Way ANOVA in Excel to test the genotypic differences of the enzymatic activities (Figure 6). Generate boxplot chart using BoxPlotR (http://shiny.chemgrid.org/boxplotr/) (Spitzer et al., 2014).
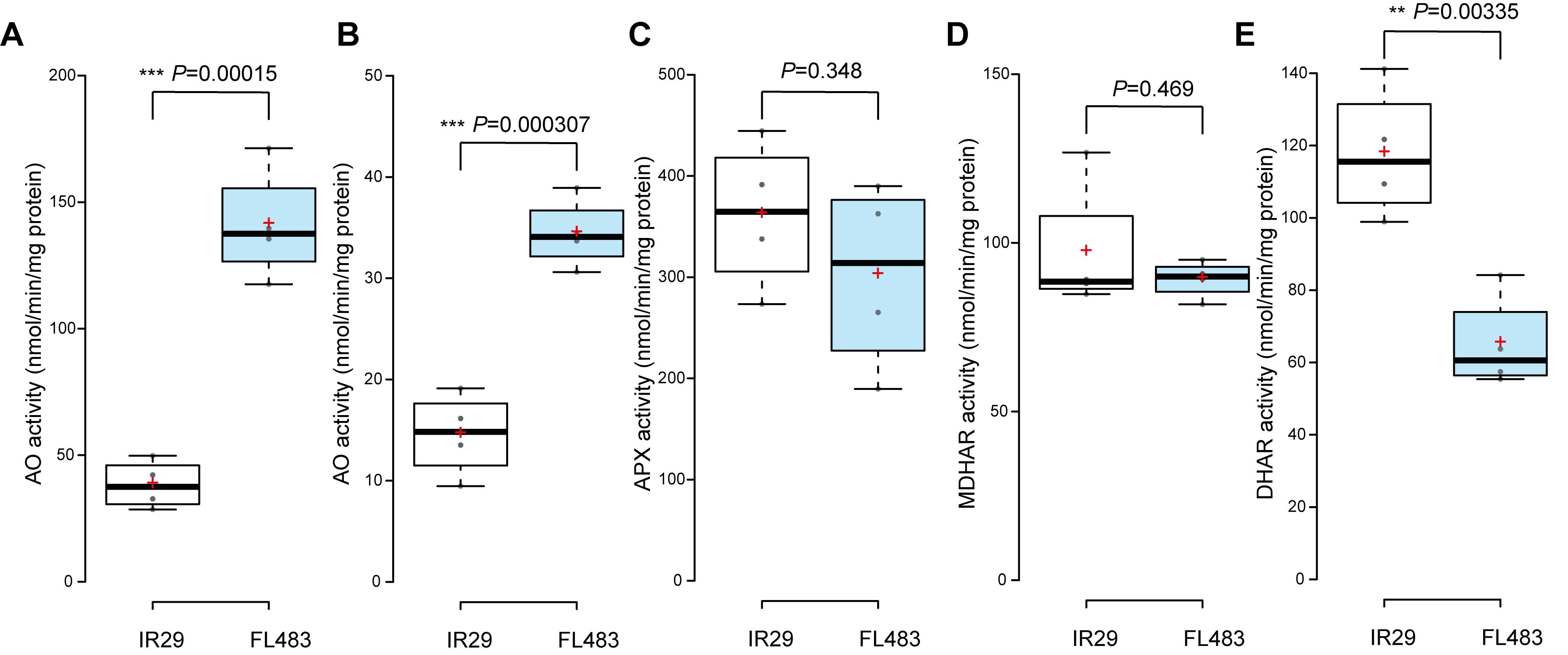
Figure 6. Ascorbate-turnover related enzymes activities in IR29 and FL483. (A) AO soluble fraction, (B) AO ionically bound fraction, (C) APX, (D) MDHAR, and (E) DHAR activities were measured in rice shoots. Data were presented as boxplots, and the red plus symbol indicates the mean value of replicated samples (N = 4). One-way ANOVA was used to test the significance level of difference between IR29 and FL483. AO, ascorbate oxidase; APX, ascorbate peroxidase; MDHAR, monodehydroascorbate reductase; DHAR, dehydroascorbate reductase. **, P < 0.01; ***, P < 0.001.
Notes
Always keep the ground samples and plant extracts under cool conditions. In the process of weighing, pre-chill the centrifuge tubes and micro-spatulas with liquid nitrogen. Grind the plant materials thoroughly to a fine powder to ensure full extraction. Add extraction buffer to the samples immediately after weighing and keep the extraction mixture on ice throughout the analysis.
Absorbance measurement requires a clear solution without any debris. Carefully transfer the supernatant without disturbing the pellets; an additional 1 min of centrifugation can improve the clearness of the extracts. Please see the Procedure part.
Thoroughly mixing different reaction components in the mixture will contribute to good linear kinetics.
APX assay requires fast handling after starting the reaction. Place the microplate ready in the reader; start the measurement immediately after adding the H2O2 solution.
Always prepare MDHAR assay buffer freshly before starting the analysis.
Measuring the absorbance at 265, 290, and 340 nm wavelength requires an UV-transparent microplate. Using a non-UV transparent microplate will lead to the failure of the assay.
When calculating the protein concentration, blank the sample absorbance values using the absorbance value of 0 mg/ml BSA standard before employing the linear correlation formula, for example, shown in Figure 5.
This protocol may be applied to analyze the leaf or shoot tissues of Tobacco, Arabidopsis, Maize, spinach, and tomato plants. Grind the plant tissue into a fine powder and clear the plant extracts to remove interfering debris are critical for successful assays.
Recipes
Artificial soil for rice growth (see Table 2)
Table 2. Composition of artificial soil for rice growth.
Component Amount (30L) Product information Hawita F.-E. Typ N 20 L Hawita Gruppe GmbH, catalog number: 0104006 Profile Porous Ceramic (PPC) Greens Grade 10 L TURF Handels GmbH, catalog number: Profile Sport Osmocote Exact 3-4 M 120 g Herrman Meyer KG, catalog number: 814169 AO extraction buffer for soluble fraction, AO-SF
100 mM sodium phosphate buffer, pH 6.5.
Add 100 ml of 100 mM NaH2PO4 (13.799 g NaH2PO4·H2O dissolved in 1 L deionized H2O) to a 250 ml beaker and adjust the pH to 6.5 using 100 mM Na2HPO4 (14.196 g Na2HPO4 dissolved in 1 L deionized H2O) solution.
AO extraction buffer for ionically bound fraction, AO-IBF
100 mM sodium phosphate buffer containing 1 M NaCl, pH 6.5.
Dissolve 5.844 g NaCl in 100 ml AO extraction buffer.
AO assay buffer and substance
100 mM sodium phosphate buffer, pH 5.6.
Dissolve AsA to 2 mM in sterile distilled water; prepare freshly for each round of analysis.
APX/MDHAR extraction buffer
50 mM potassium phosphate buffer containing 1 mM EDTA and 1 mM AsA, pH 7.8.
Add 0.2 ml of EDTA stock solution (500 mM, pH 8) and 1 ml of AsA stock solution (100 mM) into 98.8 ml of 50 mM potassium phosphate buffer (pH 7.8).
APX assay buffer and substance
100 mM potassium phosphate buffer containing 0.12 mM AsA, pH 6.8.
0.03% (V/V) H2O2 solution; prepare fresh.
AO from Cucurbita sp. enzyme solution
Dissolve 1 U/µl AO in 50% glycerol prepared with 100 mM sodium phosphate buffer, pH 6.0.
MDHAR assay buffer and substance
50 mM Tris-HCl buffer containing 2.5 mM AsA and 0.125 U of ascorbate oxidase, pH 7.6; prepare freshly for each round of analysis.
1 mM NADH in 50 mM Tris-HCl buffer, pH 7.6; prepare fresh.
DHAR extraction buffer
50 mM Tris-HCl buffer containing 100 mM NaCl, 2 mM EDTA and 1 mM MgCl2, pH 7.4.
DHAR assay buffer and substances
50 mM potassium phosphate buffer, pH 6.5.
5 mM DHA dissolved in assay buffer; prepare fresh.
50 mM reduced GSH dissolved in assay buffer; prepare fresh.
Acknowledgments
This protocol is established based on the Methods section in our previous publication (Wu et al., 2017) with minor modifications.
Competing interests
The authors declare no financial or non-financial competing interests.
References
- Das, P., Lakra, N., Nutan, K. K., Singla-Pareek, S. L., and Pareek, A. (2015). Pot Level Drought Stress Tolerance Assay in Tobacco through Plant Phenotyping and Antioxidant Assay. Bio-protocol 5(19): e1605.
- Gallie, D. R. (2013). The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 64(2): 433-443.
- Hossain, M. A., Nakano, Y., and Asada, K. (1984). Monodehydroascorbate Reductase in Spinach Chloroplasts and Its Participation in Regeneration of Ascorbate for Scavenging Hydrogen Peroxide. Plant Cell Physiol 25(3): 385-395.
- Johnston, E. J., Rylott, E. L., Beynon, E., Lorenz, A., Chechik, V., and Bruce, N. C. (2015). Monodehydroascorbate reductase mediates TNT toxicity in plants. Science 349(6252): 1072-1075.
- Lim, S., Kim, Y. H., Kim, S. H., Kwon, S.-Y., Lee, H. S., Kim, J. S., Cho, K. Y., Paek, K. Y., and Kwak, S. S. (2007). Enhanced tolerance of transgenic sweet potato plants that express both CuZnSOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Mol Breeding 19(3): 227-239.
- Pignocchi, C., Fletcher, J. M., Wilkinson, J. E., Barnes, J. D., and Foyer, C. H. (2003). The function of ascorbate oxidase in tobacco. Plant Physiol 132(3): 1631-1641.
- Smirnoff, N., and Wheeler, G. L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35(4): 291-314.
- Spitzer, M., Wildenhain, J., Rappsilber, J., and Tyers, M. (2014). BoxPlotR: a web tool for generation of box plots. Nat Methods 11(2): 121-122.
- Stahl, R. L., Liebes, L. F., Farber, C. M., and Silber, R. (1983). A spectrophotometric assay for dehydroascorbate reductase. Anall Biochem 131(2): 341-344.
- Stevens, R., Page, D., Gouble, B., Garchery, C., Zamir, D., and Causse, M. (2008). Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ 31(8): 1086-1096.
- Wu, L. B., Ueda, Y., Lai, S. K., and Frei, M. (2017). Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Environ 40(4): 570-584.
- Yan, H., Li, Q., Park, S. C., Wang, X., Liu, Y. J., Zhang, Y. G., Tang, W., Kou, M., and Ma, D. F. (2016). Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol Biochem 109: 20-27.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wu, L. B., Feng, Y., Zeibig, F., Alam, M. S. and Frei, M. (2021). High Throughput Analyses of Ascorbate-turnover Enzyme Activities in Rice (Oryza sativa L.) Seedlings. Bio-protocol 11(20): e4190. DOI: 10.21769/BioProtoc.4190.
Category
Plant Science > Plant biochemistry > Protein > Activity
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









