- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluating Baseline and Sensitised Heat Nociception in Adult Drosophila
Published: Vol 11, Iss 13, Jul 5, 2021 DOI: 10.21769/BioProtoc.4079 Views: 3358
Reviewed by: Sunanda MarellaMadhumala K SadanandappaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Aerotaxis Assay in Caenorhabditis elegans to Study Behavioral Plasticity
Qiaochu Li [...] Karl Emanuel Busch
Aug 20, 2022 2265 Views

Infection of the Developing Central Nervous System of Drosophila by Mammalian Eukaryotic and Prokaryotic Pathogens
Billel Benmimoun [...] Pauline Spéder
Dec 5, 2022 2046 Views

Application of a Dual Optogenetic Silencing-Activation Protocol to Map Motor Neurons Driving Rolling Escape Behavior in Drosophila Larvae
Ankura Sitaula [...] Aref Zarin
Dec 5, 2024 1610 Views
Abstract
Chronic pain is a complex disease that affects a large proportion of the population. With little to no effective treatments currently available for patients, this malady presents a large burden to society. Drosophila melanogaster has been previously used to describe conserved molecular components of nociception in larvae and adults. However, adult assays tend to rely on avoidance behaviours, and whilst larval acute thermal avoidance assays exist, larvae are not best suited to a chronic pain scenario as the condition must be long-term. Therefore, an adult thermal nociception response assay was required to study injury-evoked changes in heat nociception threshold (allodynia and hyperalgesia) over time, and we describe such a protocol here. Following leg amputation, flies display increased thermal sensitivity (allodynia) to innocuous temperatures but not an increase in magnitude of response (hyperalgesia) to noxious heat. Our method allows for individualised analysis of both allodynia and hyperalgesia.
Keywords: DrosophilaBackground
Chronic pain represents a substantial burden on society. Patients with this malady often suffer and experience a reduced quality of life (Campbell and Meyer, 2006; Pfau et al., 2012). Importantly, available treatment options are ineffective for the majority of chronic pain patients (Turk et al., 2011); however, an understanding of the basic underlying biology will lead to effective therapeutics (Grosser et al., 2017).
Extensive modelling of chronic pain has been performed in rodents (Costigan et al., 2009); however, these systems are expensive, the genetics of evaluating mouse pain responses are slow, and inducing chronic pain in a significant numbers of animals is required, which can have ethical implications. In contrast, Drosophila are inexpensive to maintain, quick to raise, and have an extensive molecular toolbox that allows rapid genetic manipulation.
Much work has been done to define the underlying nociceptive machinery required for invertebrate “pain” perception (Tracey et al., 2003; Kang et al., 2010; Neely et al., 2010 and 2011; Hamoudi et al., 2018). These systems are primarily dependent on investigating nociception reflexes in the transient larval stage (Babcock et al., 2009 and 2011; Turner et al., 2016; Patel and Cox, 2017; Lopez-Bellido and Galko, 2020) or thermal avoidance assays in adult flies. The first adult Drosophila avoidance assay described involved a sealed tube, noxiously heated, and a light source at one end, which flies were prevented from reaching because of a noxious heat barrier (Manev and Dimitrijevic, 2004). An alternate acute heat nociception system relied on floating a sealed “heat” chamber on hot water, where one surface of the chamber was then rapidly heated to 46°C, while the other reached 31°C during a 4-min trial. Flies with intact heat nociception avoided the hot surface, whereas flies that could not sense heat, or those that had heat-related motor issues or other confounding phenotypes, would fail to avoid the hot surface and rapidly become incapacitated (Neely et al., 2010).
While these assays are useful for assessing a loss of acute heat nociception, they are ineffective for evaluating sensitisation of nociceptive pathways. Here, we present an adaptation of our previously described method (Khuong et al., 2019) for analysis of acute thermal nociception responses, which allows for individualised assessment of both allodynia and hyperalgesia following injury.
Materials and Reagents
Sigmacote (Sigma Aldrich, catalog number: SL2)
Falcon bacteriological Petri dish lids, sizes 35-60 mm (Falcon, Corning, catalog number: 351008)
Sandpaper
Heat Sink Compound (RS, RS Pro, catalog number: 554-311)
Organic Fine Corn Flour (HBC Trading Australia, TUN number: 19339337303842)
Molasses (Whole Body Health Company, catalog number: MOL 600)
Yeast torula type B (H.J. Langdon Co., catalog number: 45014)
Agar 750 g (H.J. Langdon Co., catalog number: 44305)
Dulux Metalshield Flat Vivid White Epoxy Enamel Spray Paint (or similar) (Dulux, catalog number: 32C04912)
Diets (see Recipes)
Equipment
Bottle 6 oz square bottom PP (Pathtech, Genesee Scientific, catalog number: 076-32-130F)
Drosophila vials, Narrow, PS (Pathtech, Genesee Scientific, catalog number: 076-32-109)
Benchtop Flowbuddy Complete (Pathtech, Genesee Scientific, catalog number: 076-59-122BC)
FitoClima 600/1200 PLH Insect Research Chamber (Aralab)
Superfine Vannas Scissors, 8 cm (World Precision Instruments, catalog number: 501778)
Viltrox LL-126VB LED light (Viltrox, catalog number: LL-126VB)
Hot/Cold plate NG (Ugo Basile, catalog number: 35150)
Testo 925 K input handheld digital thermometer (RS, testo, 0560 9250)
C920 HD pro webcam (Logitech, catalog number: 960-000764)
Aluminium plate (200 × 200 × 3 mm), manufactured in-house
Software
BORIS (Friard and Gamba, 2016, https://www.boris.unito.it/)
Ctrax (Branson et al., 2009, http://ctrax.sourceforge.net/)
any2ufmf (Branson et al., 2009, http://ctrax.sourceforge.net/any2ufmf.html)
R (R foundation, https://www.r-project.org)
R Studio (https://www.rstudio.com)
Github Repository (https://github.sydney.edu.au/jmas3890/Hot-Plate-Assay.git)
Procedure
Fly preparation and injury
Note: Aside from the physical leg amputation, control flies should be treated exactly the same as experimental flies.
Prepare fly crosses at a standard density of 20 female and 5 male flies for two days at 25°C, 65% humidity, and a 12 h light/dark cycle before removing parents.
Two days following initial pupae eclosion, place F1 progeny into containers and allow to mate for two days.
Select healthy, intact male flies on light CO2 (less than 5 L/min CO2 using a Flowbuddy, delivered through a Flypad) at a standard density in standard Drosophila vials and allow to age until flies are between 7-9 days old, flipping onto new food every 2 days.
Under light CO2, amputate the right middle leg femur using surgical scissors and also expose control uninjured animals to CO2 in parallel (Figure 1).
Place one fly per vial and allow to recover for a further 7 days, flipping onto new food every 2 days.
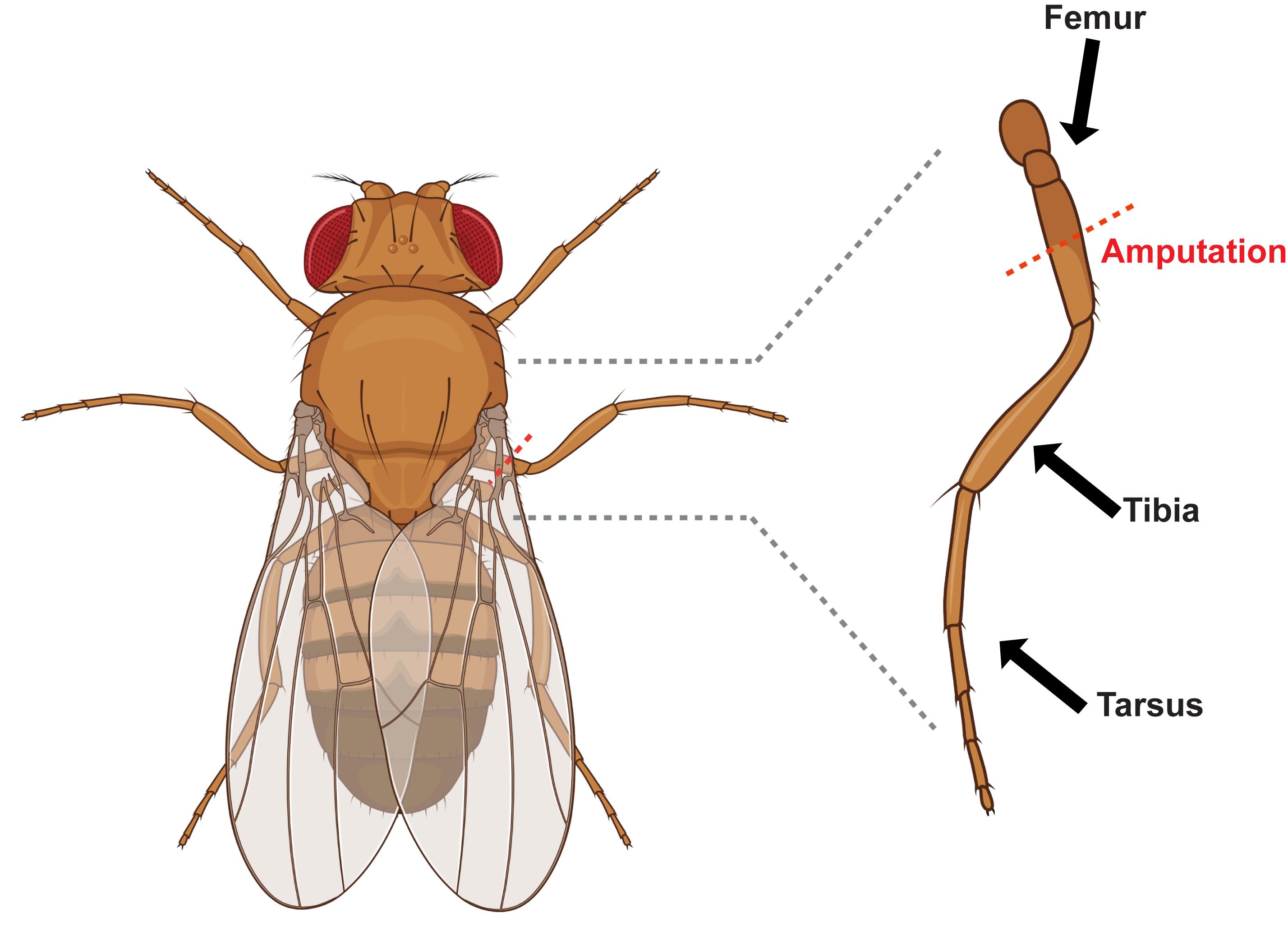
Figure 1. Leg injury model showing the location of femur amputation
Hot plate preparation
Obtain an aluminium square plate with dimensions matching the diameter of the hot plate (ensure this plate is between 1-5 mm thick to ensure efficient heat transfer).
Using thin layers, paint the aluminium plate with white matte paint.
Note: Spray paint works best for this.
Painting white is to ensure good tracking, as dark flies on a white background works best; however, if this is not a concern, skip this step.
Once the paint has dried, place the aluminium plate over the top of the Ugo Basil Hot/Cold plate (Figure 2).
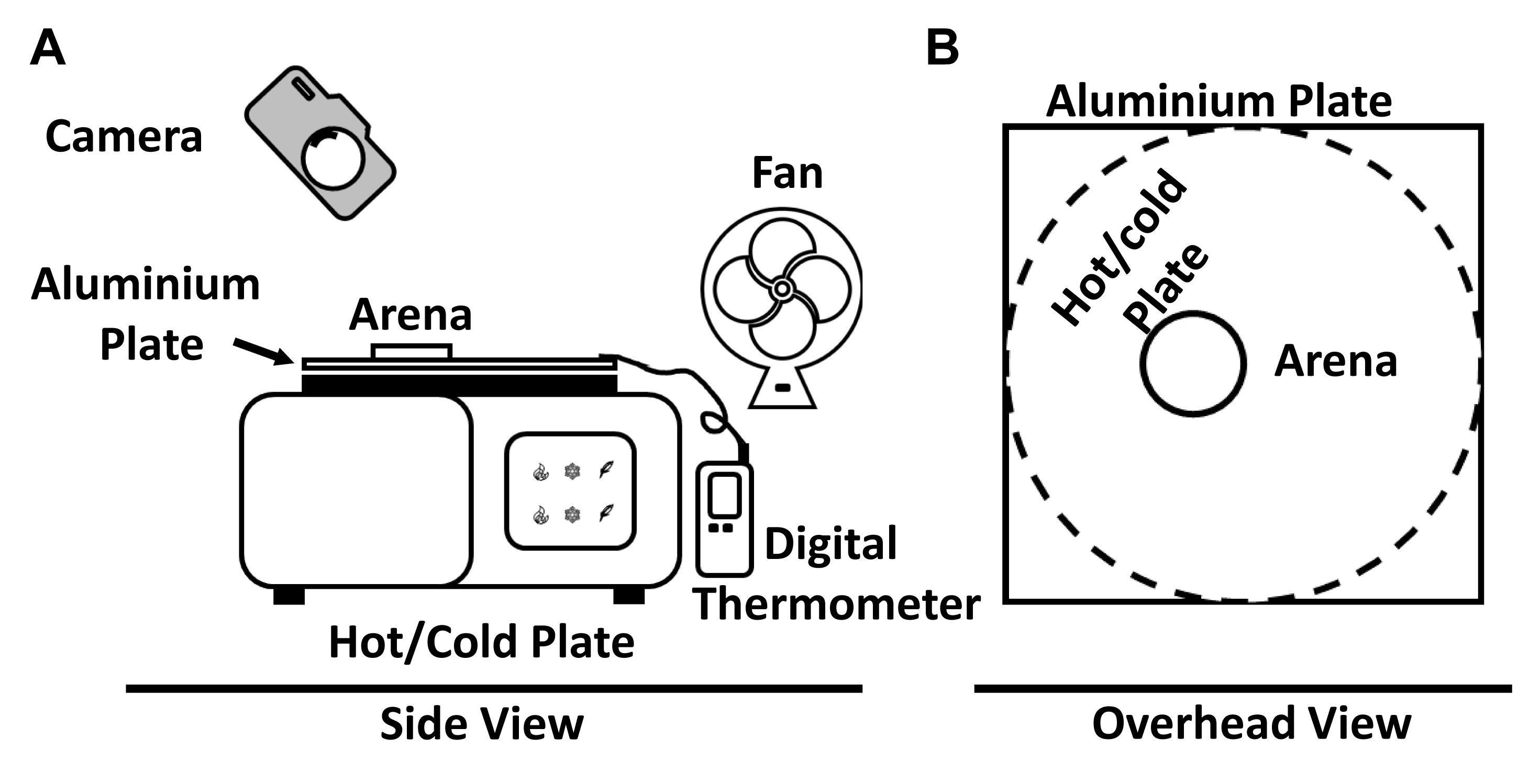
Figure 2. Arrangement of the equipment required for the hot plate assay. A. Arrangement of the Ugo Basile Hot/cold plate, Digital thermometer, Aluminium plate, Arena, Camera, and External fan. B. Simplified overhead view showing the location of the aluminium plate above the Ugo Basile hot/cold plate and arena.
Arena preparation
Take the 35-60-mm diameter Petri dish lid and using sandpaper, remove most of the walls until they are between 1-2 mm high.
Treat with Sigmacote to help stop the flies from climbing on the walls and ceiling.
Note: 1-2 coats may be necessary.
Generating a standard curve
Set the hot plate to ramp from 25°C to 50°C over 3 min.
Using a heat-transferring medium, place the tip of a digital thermal probe in the centre of the plate (or roughly where the fly will be).
Allow the plate to run and cool several times and record the temperature every 2 s for 3 min and 15 s.
Notes:
This is best done by recording the readout with a camera, C920 HD pro webcam, or similar.
This will be used later to correlate the time of the behaviour to the temperature at that time.
Hot plate assay
Set the hot plate to ramp from 25°C to 50°C over 3 min.
Place an individual fly onto the plate and quickly cover/capture with the premade arena.
Notes:
This can be done by simply flipping an individual fly onto the plate from its vial and rapidly covering with the arena (less than 30 s recovery is required for this method). Alternatively, aspirators or light anaesthesia with ice can be used (between 1-2 min and then allow double that time for recovery).
CO2 should not be used to anaesthetise flies on the day of the nociception experiment as this may affect behaviour.
Start the camera (1080 p and 30 fps) and the hot plate at the same time and record for 3 min and 15 s (195 s) before allowing the plate to cool again.
Note: Multiple external fans can be used to aid cooling.
Before beginning the next experimental replicate, ensure the plate has returned to 25°C using a thermal probe.
Repeat five times per experimental group (i.e., 5 control flies and 5 injured flies) for one technical replicate.
Technical replicates should be performed on separate days to avoid same-day experimental error.
Data analysis
Escape Response (Jump) Analysis using the BORIS behavioural Tracking Software. Detailed user manuals for the BORIS software can be found on their website (https://www.boris.unito.it/).
Create a new project and set the time format to seconds.
Create the ethogram with two behaviours. Assign these to any Key you wish; however, it is often easiest to use numbers such as 1 and 2. The behaviours we assign are:
Jump behaviour (Figure 3).
Death (this can also be useful in assessing thermal sensitivity).
Note: Here death is defined by fly curling and cessation of further movement.
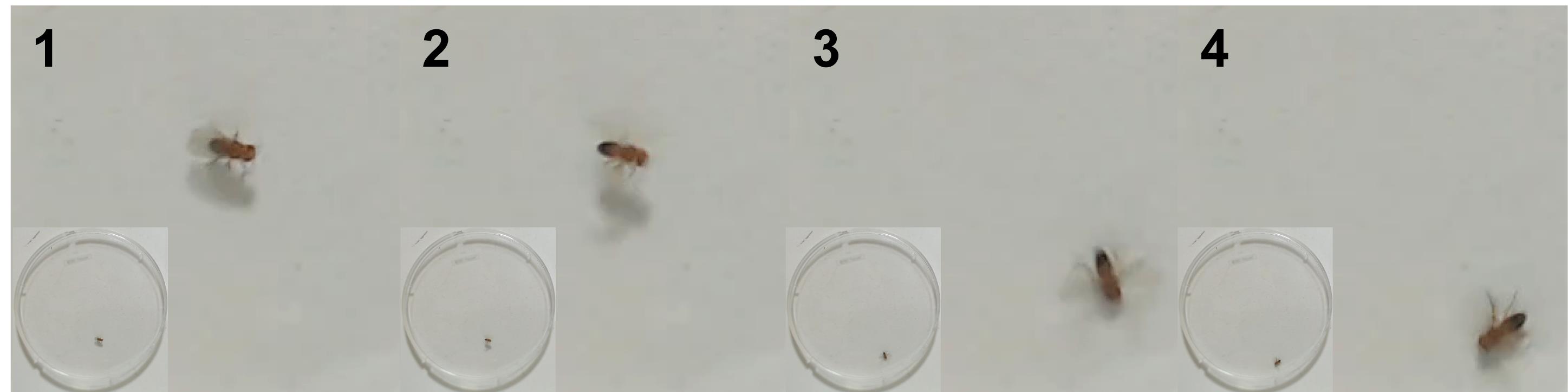
Figure 3. Consecutive frames from overhead video showing jump behaviour. Bottom left corner of each frame shows whole arena view.Save this project in the same location as the blinded videos.
Create a new observation and name it the same as the video you will be scoring.
Note: For ease of use, we randomly assign videos a sequential number.
Limit the observation time between 0.000 s and 195.000 s (the length of the behavioural assay) and then load the video to score.
Manually annotate jumps in blinded videos. Videos can be paused, slowed down, or skipped backwards to aid in behavioural annotation.
Once all videos have been scored as their own observation, use the “export events” option under the “Observation” tab to export events as tabular events and select “.csv” as the file option. Be sure to export Death events and Jump events separately and into separate folders.
Behavioural events analysis
In general, this analysis script has three main components: standard curve creation, unblinding using the Key, and jump tabulation. Here, we provide a general description of the formatting required for the standard curve and key files and how these are then utilised within the script to perform analysis.
To perform analysis, simply place all files (event .csv files, the standard curve file, and the Key) into the same folder, change the working directory to the path where this is stored (where to do this is indicated within the script), and execute the script. All scripts needed are in the GitHub Repository, as well as example files.
Standard curve.
The script will generate a curve from the average temperature at the time it was recorded. This curve is then utilised later in the script to correlate the time of the jump to the temperature at that time.
Place temperatures recorded whilst generating the standard curve in a .csv file with columns labelled (Time, Rep1, Rep2, Rep3; see example provided in the GitHub Repository).
Unblinding.
This process will take the name of the event file exported from BORIS, which should correspond to the blinded video name (i.e., 1.csv, 2.csv, etc.), and correlate that to the original name of the video as listed in the Key. For this process to work properly, it is important that the key file be formatted correctly.
The key should be formatted so that column 1 is the original name and column 2 is the blinded name (we use increasing numbers).
Note: The unblinding process will convert the blinded names into a numeric value within the script. If numbers were not used in the blinding process, this section of the script may no longer work and will need to be rewritten for your analysis. The relevant section is marked in the script contained within the GitHub Repository.
Tabulation of the jumps (Figure 4).
Tabulation is done at 1-degree bin intervals (although this can be altered and is indicated where to do so in the script).
Tabulation is started at 27.5°C and ends at 49.5°C; these temperatures can also be altered in the script. This process will include events at 49.5°C.
This script will export a file where columns show individual flies and rows show the number of escape behaviours in that bin. Bins increase by 1°C and are labelled by the lower value and not the bin centre, which will be 0.5°C higher (i.e., a bin of 27.5-28.5°C will have a label of 27.5°C and a bin centre of 28°C).
There is also a function that will tabulate total jumps, irrespective of temperature.
Note: Multiple types of analysis can then be performed, including comparison of performance at individual temperature bins or peak analysis to understand the shape and distribution of the jumps.
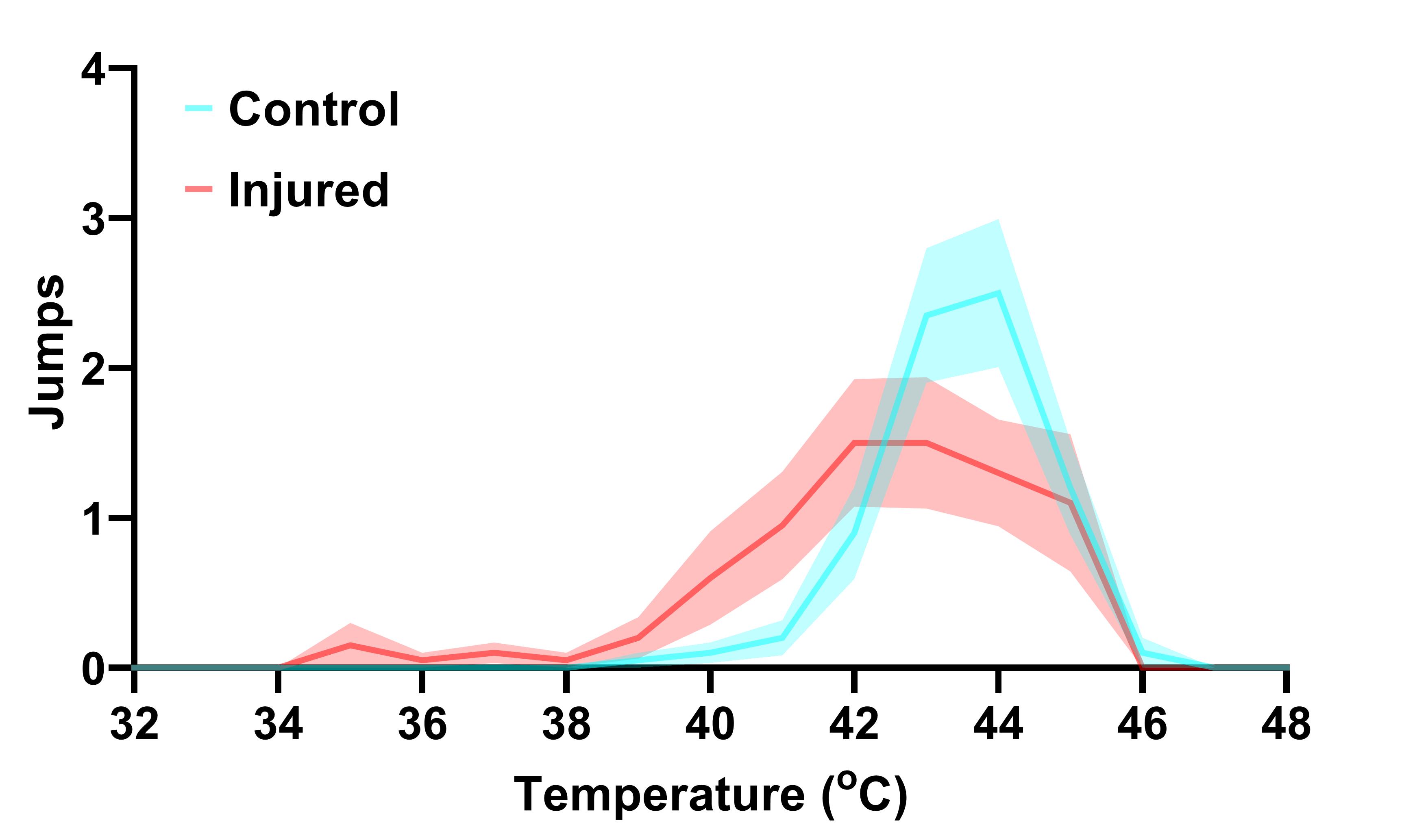
Figure 4. Jumps in control and injured Canton-S flies. 7-9-day-old male Canton-S flies underwent right femur amputation, and their escape response to thermal stimulation was recorded 7 days following initial injury. Data are presented as the mean ± SEM.
Velocity analysis (Figure 5)
Velocity analysis can be a useful tool to identify animals that may have motor issues arising from poor health (apart from the intended injury). Significant drops in velocity within experimental groups can be used to determine whether these animals should be removed from all analyses. Such animals can be identified using outlier removal assessment on the average velocity for the entire assay.
Convert video files to .ufmf format (using “any2ufmf”).
Track flies using Ctrax software or other appropriate tracking software (Detailed user guides for Ctrax and any2ufmf can be found on their website, http://ctrax.sourceforge.net/).
Note: Due to the increased velocity at high temperatures, tracking is prone to errors. Increasing both frame rate and resolution will aid but not eliminate the possibility of errors occurring. Ctrax provides a MATLAB platform to manually annotate tracking errors.
Following tracking, export as “.mat” file extension and use the R script to estimate velocities (velocities are estimated using the simple formula v = d/t). To perform analysis, place all relevant files into the same folder, change the working directory to this folder and then execute the R script.
This R script will work in a very similar fashion to the escape response script; however, it will not perform unblinding, so videos must not be blinded. It will also require similar inputs (i.e., a standard curve file and all .mat files).
Tracking errors can be removed at this step using a simple outlier removal test.
This script will output two .csv files. One file will show columns as individual flies and rows as the velocity that occurred at the corresponding temperature. The other file will show the average velocity for each individual fly.
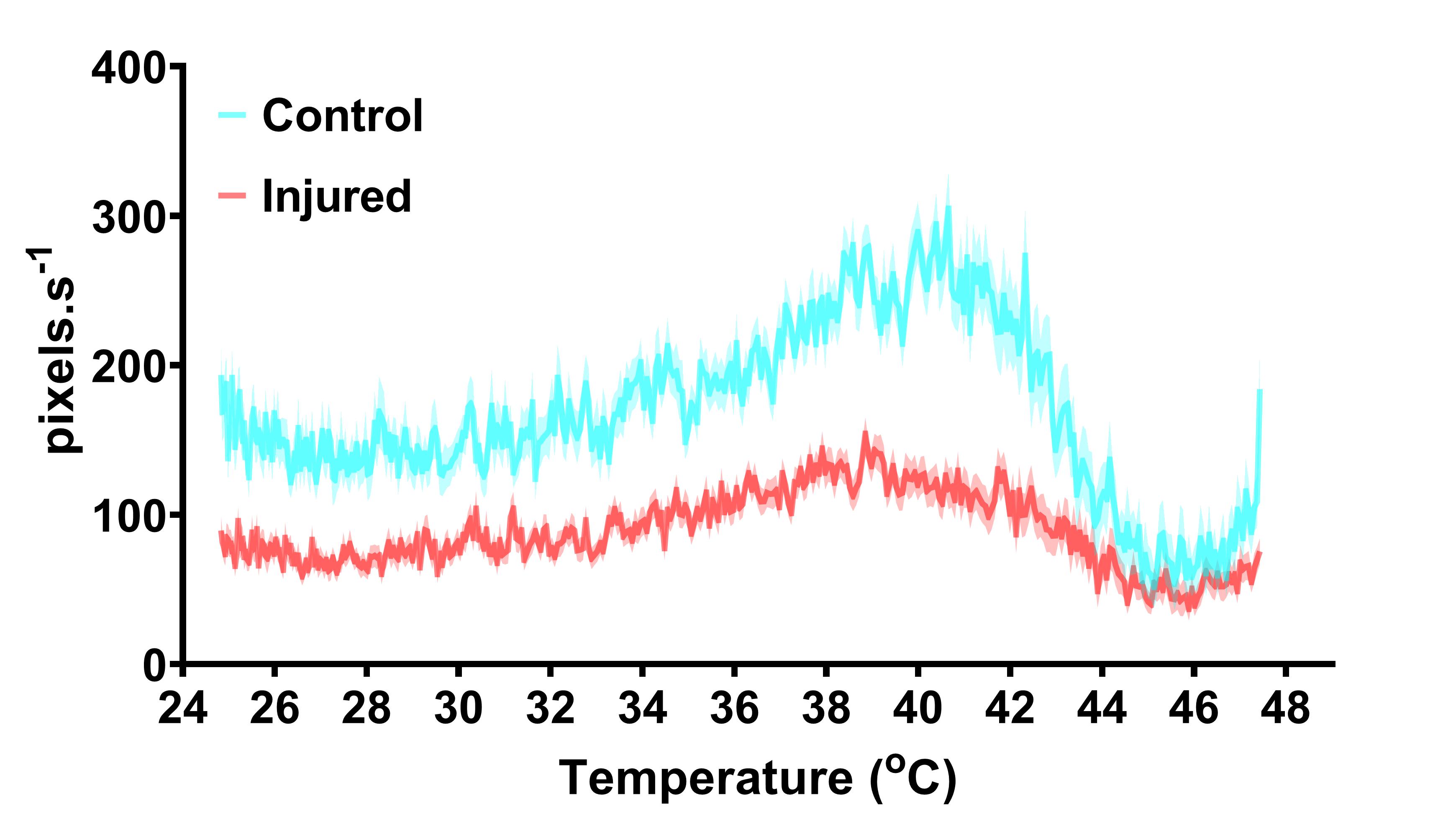
Figure 5. Velocity (pixels.s-1) in control and injured Canton-S flies. 7-9-day-old male Canton-S flies underwent right femur amputation, and their escape response to thermal stimulation was recorded 7 days following initial injury. Data are presented as the mean ± SEM.
Notes
This protocol will have a large amount of variability based on the individual experimenter. The scoring should be performed blind and by the same person for each project. Further, poor data quality will occur under poor fruit fly health, and food quality will contribute largely to this. Flies should be kept on fresh food and transferred to new food every 2-3 days. This experiment is best performed in triplicate, with three separate crosses and three separate days of recording to eliminate same-day experimental error.
Recipes
Diets
Corn flour (3.5% (w/v))
Agar (2% (w/v))
Molasses (8% (w/v))
Yeast (1.2% (w/v)) and water
Acknowledgments
This method is an adaptation of the method described by Khuong et al. (2019), DOI: 10.1126/sciadv.aaw4099.
This work was supported in part through National Health and Medical Research Council (NHMRC) project grants APP1026310, APP1029672, APP1028887, APP1046090, APP1042416, and APP1086851. G.G. Neely was supported by an NHMRC career development fellowship II CDF1111940. Finally, we thank the generosity of John Chong and Anne Chong for their financial support of work in our laboratory.
Competing interests
The authors of this work have no conflicts of interest to declare.
References
- Babcock, D. T., Landry, C. and Galko, M. J. (2009). Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol 19(10): 799-806.
- Babcock, D. T., Shi, S., Jo, J., Shaw, M., Gutstein, H. B. and Galko, M. J. (2011). Hedgehog signaling regulates nociceptive sensitization. Curr Biol 21(18): 1525-1533.
- Branson, K., Robie, A. A., Bender, J., Perona, P. and Dickinson, M. H. (2009). High-throughput ethomics in large groups of Drosophila. Nat Methods 6(6): 451-457.
- Campbell, J. N. and Meyer, R. A. (2006). Mechanisms of neuropathic pain. Neuron 52(1): 77-92.
- Costigan, M., Scholz, J. and Woolf, C. J. (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32: 1-32.
- Friard, O., Gamba, M. J. (2016). BORIS: a free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods Ecol Evol 7(11): 1325-1330.
- Grosser, T., Woolf, C. J. and FitzGerald, G. A. (2017). Time for nonaddictive relief of pain. Science 355(6329): 1026-1027.
- Hamoudi, Z., Khuong, T. M., Cole, T. and Neely, G. G. (2018). A fruit fly model for studying paclitaxel-induced peripheral neuropathy and hyperalgesia. F1000Res 7: 99.
- Kang, K., Pulver, S. R., Panzano, V. C., Chang, E. C., Griffith, L. C., Theobald, D. L. and Garrity, P. A. (2010). Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464(7288): 597-600.
- Khuong, T. M., Wang, Q. P., Manion, J., Oyston, L. J., Lau, M. T., Towler, H., Lin, Y. Q. and Neely, G. G. (2019). Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila. Sci Adv 5(7): eaaw4099.
- Lopez-Bellido, R. and Galko, M. J. (2020). An Improved Assay and Tools for Measuring Mechanical Nociception in Drosophila Larvae. J Vis Exp (164).
- Manev, H. and Dimitrijevic, N. (2004). Drosophila model for in vivo pharmacological analgesia research. Eur J Pharmacol 491(2-3): 207-208.
- Neely, G.G., Hess, A., Costigan, M., Keene, A.C., Goulas, S., Langeslag, M., Griffin, R.S., Belfer, I., Dai, F., Smith, S.B., Diatchenko, L., Gupta, V., Xia, C. P., Amann, S., Kreitz, S., Heindl-Erdmann, C., Wolz, S., Ly, C. V., Arora, S., Sarangi, R., Dan, D., Novatchkova, M., Rosenzweig, M., Gibson, D. G., Truong, D., Schramek, D., Zoranovic, T., Cronin, S. J., Angjeli, B., Brune, K., Dietzl, G., Maixner, W., Meixner, A., Thomas, W., Pospisilik, J. A., Alenius, M., Kress, M., Subramaniam, S., Garrity, P. A., Bellen, H. J., Woolf, C. J. and Penninger, J. M. (2010). A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 143(4): 628-638.
- Neely, G. G., Keene, A. C., Duchek, P., Chang, E. C., Wang, Q. P., Aksoy, Y. A., Rosenzweig, M., Costigan, M., Woolf, C. J., Garrity, P. A. and Penninger, J. M. (2011). TrpA1 regulates thermal nociception in Drosophila. PLoS One 6(8): e24343.
- Patel, A. A. and Cox, D. N. (2017). Behavioral and Functional Assays for Investigating Mechanisms of Noxious Cold Detection and Multimodal Sensory Processing in Drosophila Larvae. Bio-protocol 7(13): e2388.
- Pfau, D. B., Geber, C., Birklein, F. and Treede, R. D. (2012). Quantitative sensory testing of neuropathic pain patients: potential mechanistic and therapeutic implications. Curr Pain Headache Rep 16(3): 199-206.
- Tracey, W. D., Jr., Wilson, R. I., Laurent, G. and Benzer, S. (2003). Painless, a Drosophila gene essential for nociception. Cell 113(2): 261-273.
- Turk, D. C., Wilson, H. D. and Cahana, A. (2011). Treatment of chronic non-cancer pain. Lancet 377(9784): 2226-2235.
- Turner, H. N., Armengol, K., Patel, A. A., Himmel, N. J., Sullivan, L., Iyer, S. C., Bhattacharya, S., Iyer, E. P. R., Landry, C., Galko, M. J. and Cox, D. N. (2016). The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Curr Biol 26(23): 3116-3128.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Massingham, J. N., Baron, O. and Neely, G. G. (2021). Evaluating Baseline and Sensitised Heat Nociception in Adult Drosophila. Bio-protocol 11(13): e4079. DOI: 10.21769/BioProtoc.4079.
- Khuong, T. M., Wang, Q. P., Manion, J., Oyston, L. J., Lau, M. T., Towler, H., Lin, Y. Q. and Neely, G. G. (2019). Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila.Sci Adv 5(7): eaaw4099.
Category
Neuroscience > Sensory and motor systems > Animal model
Neuroscience > Nervous system disorders
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








