- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo Optical Access to Olfactory Sensory Neurons in the Mouse Olfactory Epithelium
Published: Vol 11, Iss 12, Jun 20, 2021 DOI: 10.21769/BioProtoc.4055 Views: 4126
Reviewed by: Joseph ZakWei WangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Lipid-Mediated Sequential Recruitment of Proteins Via Dual SLIPT and Dual SLIPTNVOC in Live Cells
Kristina V. Bayer and Richard Wombacher
Nov 5, 2025 1601 Views

Monitoring of Sperm-Independent Calcium Oscillations in Immature Oocytes of Mice
Sae Horiike [...] Hidehiko Ogawa
Feb 5, 2026 106 Views

High Content In Vitro Survival Assay of Cortical Neurons
Paolo V. Fioretti [...] Manuela Basso
Feb 5, 2026 111 Views
Abstract
In neuroscience, it is fundamental to understand how sensory stimuli are translated into neural activity at the entry point of sensory systems. In the olfactory system, odorants inhaled into the nasal cavity are detected by ~1,000 types of odorant receptors (ORs) that are expressed by olfactory sensory neurons (OSNs). Since each OSN expresses only one type of odorant receptor, the odor-evoked responses reflect the interaction between odorants and the expressed OR. The responses of OSN somata are often measured by calcium imaging and electrophysiological techniques; however, previous techniques require tissue dissection or cell dissociation, rendering it difficult to investigate physiological responses. Here, we describe a protocol that allows us to observe odor-evoked responses of individual OSN somata in the mouse olfactory epithelium in vivo. Two-photon excitation through the thinned skull enables highly-sensitive calcium imaging using a genetically encoded calcium indicator, GCaMP. Recording of odor-evoked responses in OSN somata in freely breathing mice will be fundamental to understanding how odor information is processed at the periphery and higher circuits in the brain.
Keywords: Olfactory systemBackground
Animals recognize their environmental cues using sensory systems. The mammalian olfactory system is able to detect and discriminate a large repertoire of odorants. Odorants inhaled into the nasal cavity are detected by ~1,000 types of odorant receptors (ORs) expressed by olfactory sensory neurons (OSNs) in the olfactory epithelium (OE) of mice. Since each OSN expresses only one type of OR, the odor-evoked responses reflect the interaction between odorants and the expressed OR. To understand how odor information is translated into neural activity at the entry point of the olfactory system, it is important to study OSN responses in the olfactory epithelium in vivo.
The responses of OSN somata are often measured by calcium imaging and electrophysiological techniques (Maue and Dionne, 1987; Cygnar et al., 2010; Jarriault and Grosmaitre, 2015; Zhang, 2018); however, previous techniques require tissue dissection or cell dissociation, rendering it difficult to investigate physiological responses. Electroolfactograms can be used in vivo, but they cannot distinguish single-cell activities.
Here, we describe a protocol that allowed us to observe the odor-evoked responses of individual OSN somata in the OE in vivo (Iwata et al., 2017; Inagaki et al., 2020; Zak et al., 2020). Two-photon excitation through the thinned skull enables highly-sensitive calcium imaging using a genetically encoded calcium indicator, GCaMP (Yang et al., 2010). The preparation for in vivo imaging is simple and usually completed within 1 h. This method may apply not only to calcium imaging but also to other types of fluorescence imaging of OSNs.
Materials and Reagents
1.5 ml plastic tubes (Bio-bik, catalog number: CF-0150)
27 G needles for injection (Terumo, catalog number: NN-2719S)
1 ml syringe (Terumo, catalog number: 170215)
50 ml centrifuge tubes (Greiner, catalog number: 227261)
Teflon tube (Chiyoda, catalog number: TF-4-10)
KimWipes (Crecia, catalog number: S-200)
Cotton buds (Suzuran, catalog number: 102046)
Toothpicks (Yanagi, catalog number: J-613)
Disposable balance tray (Bio-bik, catalog number: AS-DS)
Cement solution (GC, Product name: Unifast II liquid 100 g)
Cement powder (GC, Product name: Unifast II powder A3 35 g)
Saline (Otsuka, catalog number: 3311401A7028)
Ketamine (Daiichi-Sankyo, catalog number: S9-019780)
Xylazine (Bayer, Product name: Rompun 2% w/v solution for injection 25 ml)
Vaseline (Wako, catalog number: 227-01211)
70% ethanol (Shinwa, catalog number: WK2-75)
Superglue (Sankyo, Product name: aron alpha A 0.5 g × 5)
Kwil-sil (WPI, catalog number: KWIK-SIL)
Valeraldehyde (Tokyo Chemical Industry, catalog number: V0001)
Mineral oil (Sigma, catalog number: M5310-500 ML)
Phosphate-buffered saline (PBS)
Mouse:
The OSN-specific GCaMP transgenic mouse line, OSN-GCaMP3 (OMP-tTA; TRE-GCaMP3 compound heterozygous bacterial artificial chromosome transgenic mice, 8-16 weeks of age) was used (Iwata et al., 2017; Inagaki et al., 2020). OMP-tTA (Accession# CDB0506T) and TRE-GCaMP3 (Accession# CDB0505T) are available from RIKEN (http://www2.clst.riken.jp/arg/index.html).
Note: Transgenic mouse lines expressing any indicators could be used, but sparsely and brightly labeled lines are preferred so that you can easily distinguish OSN responses. In OSN-GCaMP3 mice, GCaMP3 is expressed in 57.9% of the total OSNs (Iwata et al., 2017). A mouse line based on the Tet-system may be suitable for OE imaging in terms of labeling density and fluorescence intensity.
Equipment
Micropipette (Gilson, model: P200 and P1000)
Heating pad (Natsume, catalog number: KN-475-3-40)
Forceps (KFI, catalog number: 1-9749-32)
Fine forceps (Ideal-tek, catalog number: 91-2427)
Fine scissors (Mizuho, catalog number: 04-001-13)
Fluorescent stereomicroscope (Leica, model: M205 C)
Note: An epifluorescence microscope is useful for assessing the thickness of the skull above the OE (detailed below).
External light source for fluorescence excitation (Leica, model: EL6000)
Filter cube (Leica, model: GFP)
Head holder for surgery (Narishige, model: SG-4N)
Custom-made aluminum nose bar (Figure 1)
Note: We installed a custom-made nose bar to Narishige SG-4N (Figure 1A), as the original nose bar was too long and prevented surgical access to the OE. The size should be adjusted to your head holder and to make the skull over the OE accessible during surgery (Figure 1C). Any head holders can be used as long as the OE is accessible for surgery.
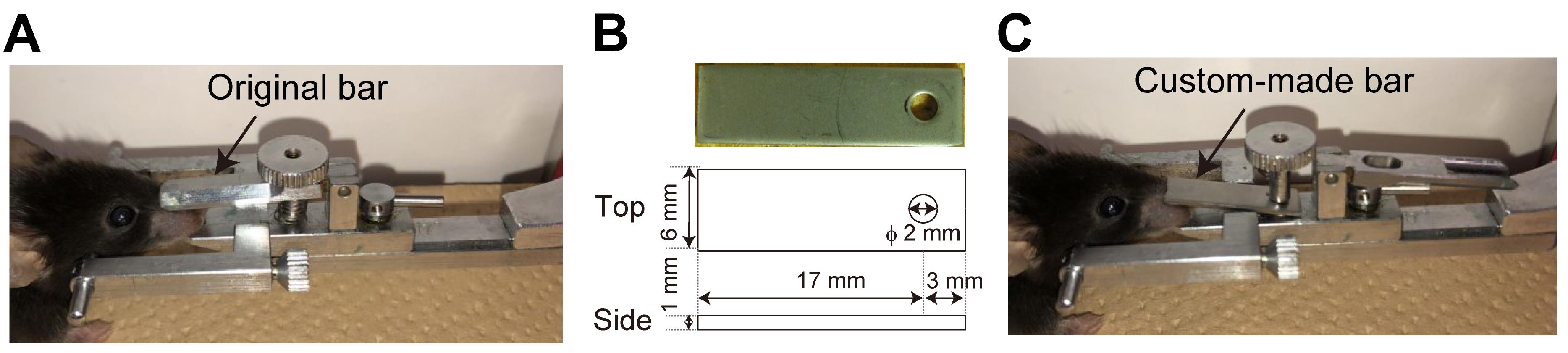
Figure 1. Installation of a custom-made nose bar to a head holder. A. An original nose bar of a head holder. B. The design of a custom-made nose bar. C. The custom-made nose bar is installed into SG-4N.Custom-made aluminum head bar (4 × 22 mm)
Note: The head bar was designed for a custom-built head holder as described in a previous study (Guo et al., 2014).
Custom-built head holder for imaging
Note: The head holder was built as described previously (Guo et al., 2014). Any head-holding system can be used for this protocol if the OE is accessible for in vivo imaging.
Φ1 mm drill tip (Meisinger, catalog number: ST1 HP010)
Dental drill (Leutor, model: LP-120)
Dust blower (UN, catalog number: UN-1321)
Two-photon microscope (Olympus, model: FV1000MPE)
Fluoview software (Olympus, model: FV10-ASW)
25× objective lens (Olympus, model: XLPLN25XWMP)
Custom-built olfactometer
Note: The design of the olfactometers has been described elsewhere (Slotnick and Restrepo, 2005; Burton et al., 2019). Briefly, the olfactometer consists of an air pump (AS ONE, catalog number: 1-7482-11), activated charcoal filter (Advantec, model: TCC-A1-S0C0 and 1TS-B), and flowmeters (Kofloc, model: RK-1250)]
Procedure
Prepare a head holder for surgery. We used a combination of a commercial head holder and a custom-made nose bar to make the OE region accessible for surgery (Figure 1).
Anesthetize a mouse using a ketamine/xylazine cocktail in saline (80 mg/kg and 16 mg/kg for ketamine and xylazine, respectively). Inject the ketamine/xylazine cocktail intraperitoneally using a 1-ml syringe and 27 G needle. During surgery, the depth of anesthesia needs to be assessed by the toe-pinch reflex, and supplemental doses should be administered when necessary.
Hold the head under a fluorescent stereomicroscope using the head holder.
Cover the eyes with Vaseline using a cotton bud to prevent drying.
Apply 70% ethanol on the head.
Note: This step is required to sterilize the surgical site and to remove hairs in the next step.
Remove the scalp together with hairs using scissors and forceps (Figure 2A).
Note: The scalp needs to be extensively removed to the back of the head to attach a custom-made head bar at the later step.
Note: The hair can be shaved with a razor beforehand.
Carefully remove the periosteum from the skull with forceps.
Apply superglue to the periphery of the surgical site to prevent the scalp from being caught up by the rotation of the drill.
Note: Superglue can harden quickly when PBS is overlaid. A used drill tip is useful for application of superglue and PBS.
Carefully thin the skull over the OE using a dental drill (Φ 1 mm drill tip, 5,000-10,000 rpm) (Figure 2A-2D, Video 1).
Note: The dorsal and rostral parts of the D zone (zone 1) and the dorsolateral part of the V zone (zone 4) can be imaged (Figure 2B). Other parts are difficult to drill due to the presence of a lot of blood vessels. To avoid overheating, do not continuously thin the same area of the skull. See Yang et al. (2010) for additional tips on thinned skull preparations.
Video 1. Thinned-skull preparation for OE imaging. A drill tip was lightly touched to the skull and moved horizontally.Blow away the skull shavings with a dust blower (Video 1).
Apply a small amount of PBS on the thinned skull and check if the blood vessels and fluorescence of OSN somata can be clearly observed (Figure 2D-2F).
Continue thinning until the fluorescence of OSN somata is observed (Figure 2F, arrows).
Note: You can also estimate the thickness of the skull based on the stiffness. If it is thin enough, the skull sinks a little when touched lightly with forceps.
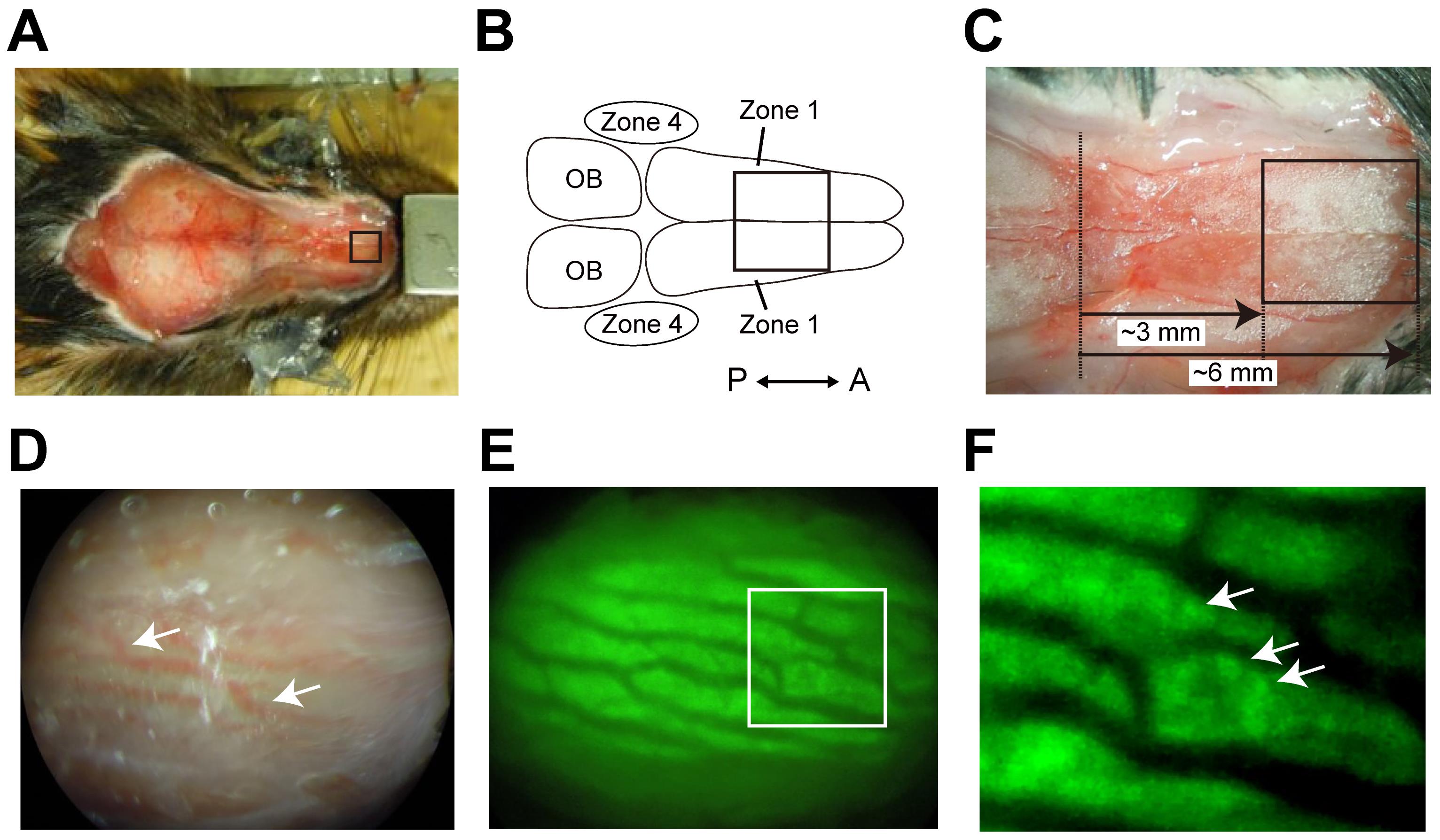
Figure 2. Thinned-skull preparation for in vivo imaging. A. The dorsal scalp was removed from the head. B. The dorsal and rostral parts of the D zone (zone 1) and the dorsolateral part of the V zone (zone 4) in the OE can be imaged. C. A close-up picture of the imaging area over zone 1 in the OE. The skull was thinned in the boxed area (A-C, 3-6 mm from the anterior edge of the olfactory bulb in the 12-week-old male mice). D. Brightfield image of zone 1 in the right OE taken through the thinned skull. The blood vessels should be clearly observed if the skull is sufficiently thinned (white arrows). E. A fluorescence image of zone 1 in the right OE taken by a fluorescent stereomicroscope. F. A close-up image of the square region indicated in (E). Arrows indicate fluorescence from OSN somata.Adjust the head angle to make sure that the dorsal surface of the OE is perpendicular to the light path.
Apply superglue to the surface of the skull outside the imaging area to make a scaffold for the attachment of a custom-made head bar.
Place ~0.3 g cement powder into a disposable balance tray. Pour ~0.3 ml cement solution onto the powder using a micropipette. Mix the powder and solution with a toothpick immediately to make the dental cement (Figure 3A).
Note: Larger amounts of solution may dissolve the plastic tray. In that case, you can use a small silicone bowl instead.
Attach a custom-made head bar perpendicular to the light path using dental cement (Figure 3B).
Apply Kwik-sil at the periphery of the imaging area. PBS can be kept here to image using a water-immersion objective lens (Figure 3C).
Fix the head under a two-photon microscope using the head bar and a custom-built head holder (Figure 3D) (Guo et al., 2014).
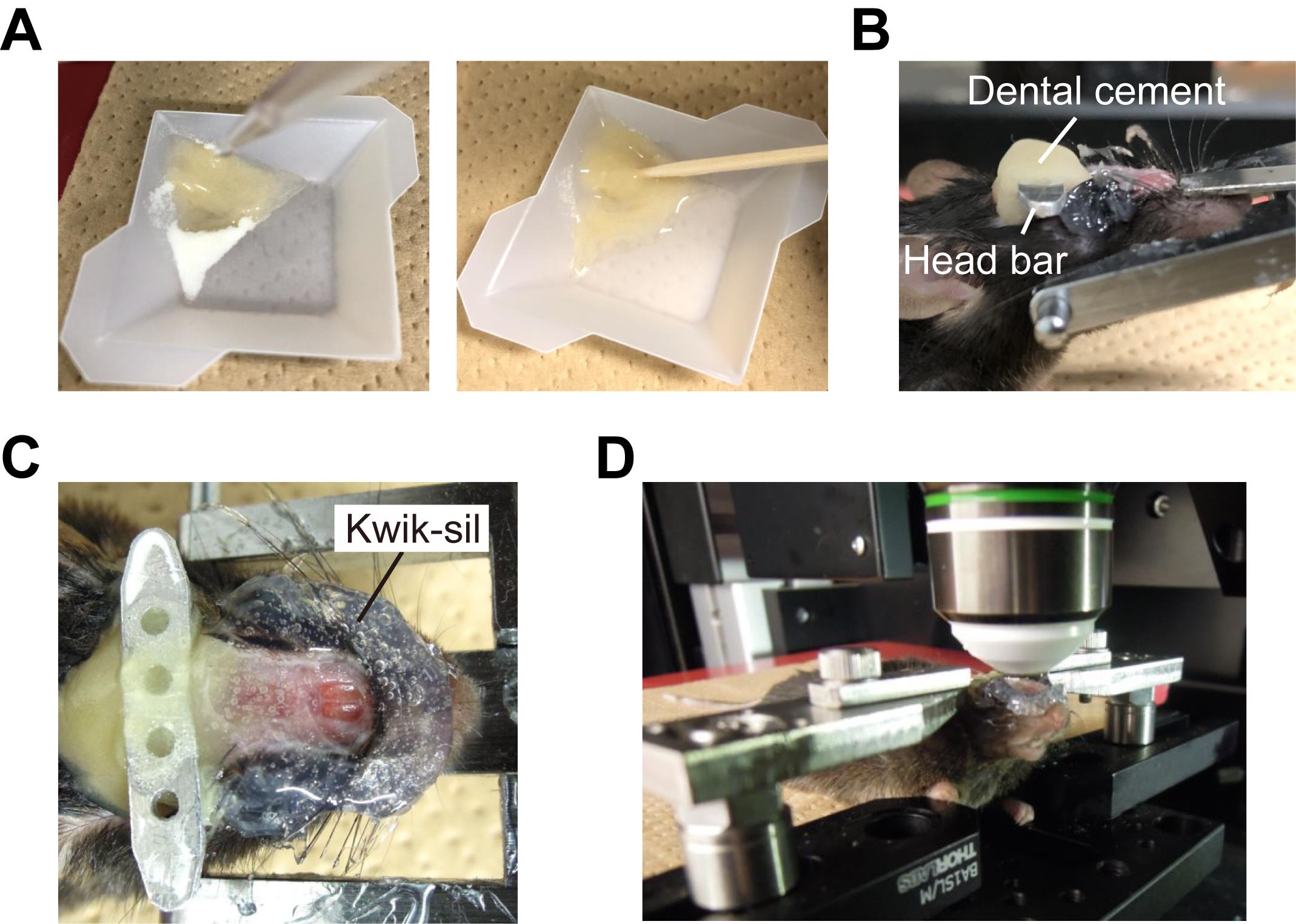
Figure 3. Head-fixation under a two-photon microscope. A. Dental cement before (left) and after (right) mixing with a toothpick. B. A custom-made head bar was attached with dental cement perpendicular to the optical axis of the subsequent in vivo imaging. C. Kwik-sil was applied to the periphery of the imaging area to retain PBS during imaging. D. The imaging area was placed under an objective lens using a custom-made head holder.Perform two-photon imaging of odor-evoked responses in OSN somata with a custom-built olfactometer (Figure 4; Video 2). In this example, valeraldehyde was diluted at a concentration of 0.5% v/v in 1 ml mineral oil and soaked in a Kimwipe in a 50-ml centrifuge tube. Saturated odor vapor in the centrifuge tube was delivered to the nose via a Teflon tube at 1 L/min.
Notes:
The 50-ml centrifuge tube and Teflon tube should be replaced every time the odors are changed to avoid cross-contamination of the odors.
We have never performed chronic imaging, but it may be possible (see also Zak et al., 2020).
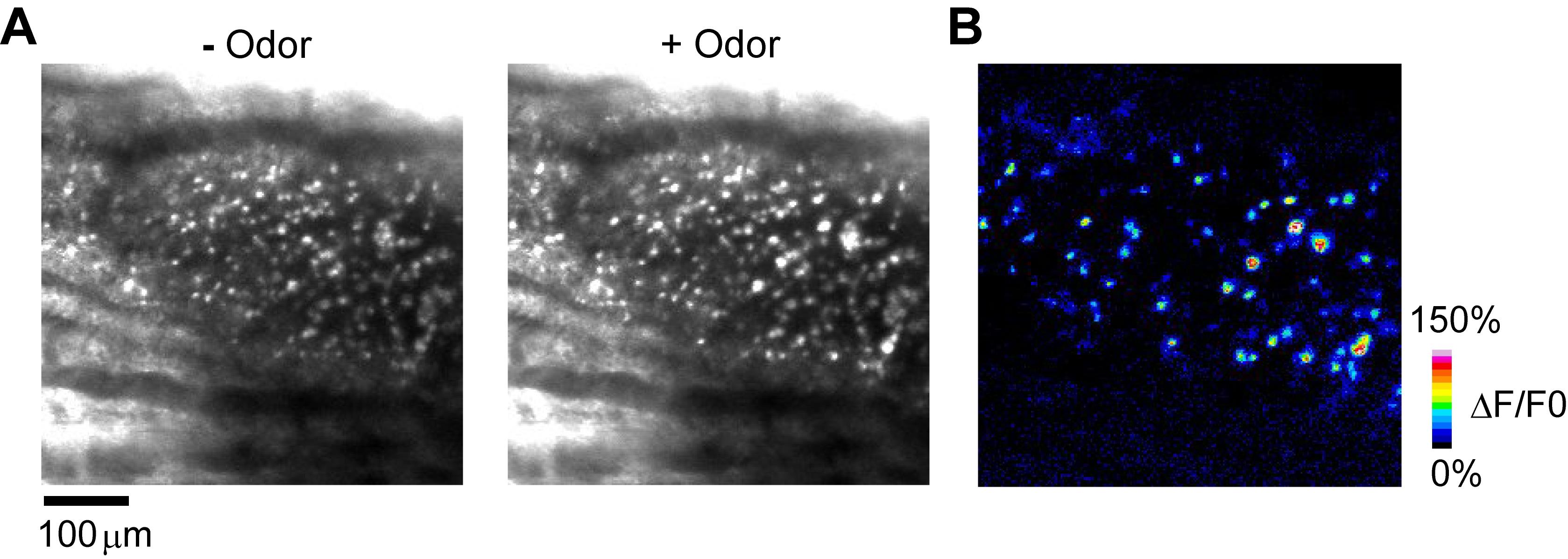
Figure 4. Two-photon calcium imaging of OSN somata. A. GCaMP3 fluorescence from OSN somata before (left) and during (right) odor stimulation. B. A pseudo-colored ∆F/F0 image of OSN somata responses to 0.5% valeraldehyde (see Video 2).Video 2. In vivo two-photon imaging of odor responses at the OSN somata in the OE. 0.5% valeraldehyde was delivered to the nose from 10 to 15 s. Gray-scale images show the fluorescence (pixel intensities).
Acknowledgments
This work was supported by grants from the PRESTO and CREST programs of the Japan Science and Technology Agency (JST), Japan (T.I.), the JSPS KAKENHI, Japan (grant numbers JP23680038, JP15H05572, JP15K14336, JP16K14568, JP16H06456, JP17H06261, and JP21H00205 to T.I., JP15K18353 to R.I., and JP21H02140 to S.I.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research, intramural grant from RIKEN Center for Developmental Biology (T.I.), and Grant-in-Aid for JSPS Research Fellow, Japan (JP15J08987 to R.I. and JP18J00899 to S.I.). We thank M.N. Leiwe for comments on the manuscript. This protocol has been used in our research (Iwata et al., 2017; Inagaki et al., 2020).
Competing interests
There are no conflicts of interests or competing financial interests.
Ethics
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Kyushu University (#A19-054, approved from 04/01/19 to 03/31/21).
References
- Burton, S. D., Wipfel, M., Guo, M., Eiting, T. P. and Wachowiak, M. (2019). A Novel Olfactometer for Efficient and Flexible Odorant Delivery. Chem Senses 44(3): 173-188.
- Cygnar, K.D., Stephan, A.B. and Zhao, H. (2010). Analyzing responses of mouse olfactory sensory neurons using the air-phase electroolfactogram recording. J Vis Exp (37): 1850.
- Guo, Z. V., Hires, S. A., Li, N., O'Connor, D. H., Komiyama, T., Ophir, E., Huber, D., Bonardi, C., Morandell, K., Gutnisky, D., Peron, S., Xu, N. L., Cox, J. and Svoboda, K. (2014). Procedures for behavioral experiments in head-fixed mice. PLoS One 9(2): e88678.
- Inagaki, S., Iwata, R., Iwamoto, M. and Imai, T. (2020). Widespread inhibition, antagonism, and synergy in mouse olfactory sensory neurons in vivo. Cell Rep 31(13): 107814.
- Iwata, R., Kiyonari, H. and Imai, T. (2017). Mechanosensory-based phase coding of odor identity in the olfactory bulb. Neuron 96(5): 1139-1152 e1137.
- Jarriault, D. and Grosmaitre, X. (2015). Perforated Patch-clamp Recording of Mouse Olfactory Sensory Neurons in Intact Neuroepithelium: Functional Analysis of Neurons Expressing an Identified Odorant Receptor. J Vis Exp 101: e52652.
- Maue, R. A. and Dionne, V.E. (1987). Patch-clamp studies of isolated mouse olfactory receptor neurons. J Gen Physiol 90(1): 95-125.
- Slotnick, B. and Restrepo, D. (2005). Olfactometry with mice. Curr Protoc Neurosci Chapter 8: Unit 820.
- Yang, G., Pan, F., Parkhurst, C.N., Grutzendler, J. and Gan, W.B. (2010). Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc 5(2): 201-208.
- Zak, J. D., Reddy, G., Vergassola, M. and Murthy, V. N. (2020). Antagonistic odor interactions in olfactory sensory neurons are widespread in freely breathing mice. Nat Commun 11(1):3350.
- Zhang, C. (2018). Calcium Imaging of Individual Olfactory Sensory Neurons from Intact Olfactory Turbinates. Methods Mol Biol 1820: 57-68.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Inagaki, S., Iwata, R. and Imai, T. (2021). In vivo Optical Access to Olfactory Sensory Neurons in the Mouse Olfactory Epithelium. Bio-protocol 11(12): e4055. DOI: 10.21769/BioProtoc.4055.
Category
Neuroscience > Behavioral neuroscience > Olfaction
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











