- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Objective Quantitation of Focal Sweating Areas Using a Mouse Sweat-assay Model
Published: Vol 11, Iss 11, Jun 5, 2021 DOI: 10.21769/BioProtoc.4047 Views: 3826
Reviewed by: Karem A CourtArvind Kumar Singh ChandelKuo-Ching "KC" Mei

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Whole-mount Staining of Mouse Diaphragm Neuromuscular Junctions
Rula Sha [...] Ying Feng
Nov 5, 2021 4649 Views

Operant Self-medication for Assessment of Spontaneous Pain Relief and Drug Abuse Liability in Mouse Models of Chronic Pain
David Cabañero [...] Rafael Maldonado
Mar 5, 2022 3055 Views

Developing a Ministroke Model in Mouse Barrel Cortex
Song Wang [...] Yihan Wu
Mar 20, 2025 2350 Views
Abstract
In vivo sweat quantitation assays are required for the development of drugs for the management of focal hyperhidrosis before clinical trials; however, in vivo assays, particularly mouse models, are rare. Even in sweat assays using mice, sweating is quantitated by manually counting the number of sweating spots, which can contribute to various errors owing to arbitrary judgment. In this study, we developed a mouse sweat-assay model and a method for quantitating the amount of sweating to remove possible errors. The use of the iodine–starch test in the castor oil-covered hind footpad skin of anesthetized mice resulted in the sweating area being stained blue-black. After the anesthesia and treatment with drugs (pilocarpine, glycopyrrolate, botulinum neurotoxin, myricetin, and myricetin-loaded lipid nanoparticles), the remaining area of the footpad skin was eliminated from the acquired footpad images using ImageJ. Blue pixels extracted from the footpad image are automatically adjusted using the Phansalkar method, where the percentage of the blue area was determined based on the whole hind footpad skin area, finally indicating the percentage of the sweating area. Using this mouse model and analysis for sweat assays, a clear difference between the control group and antiperspirant-administered group was observed with respect to the sweating area % with no error. In conclusion, this assay can be used as a preclinical tool to screen potential antiperspirant drugs.
Graphic abstract:
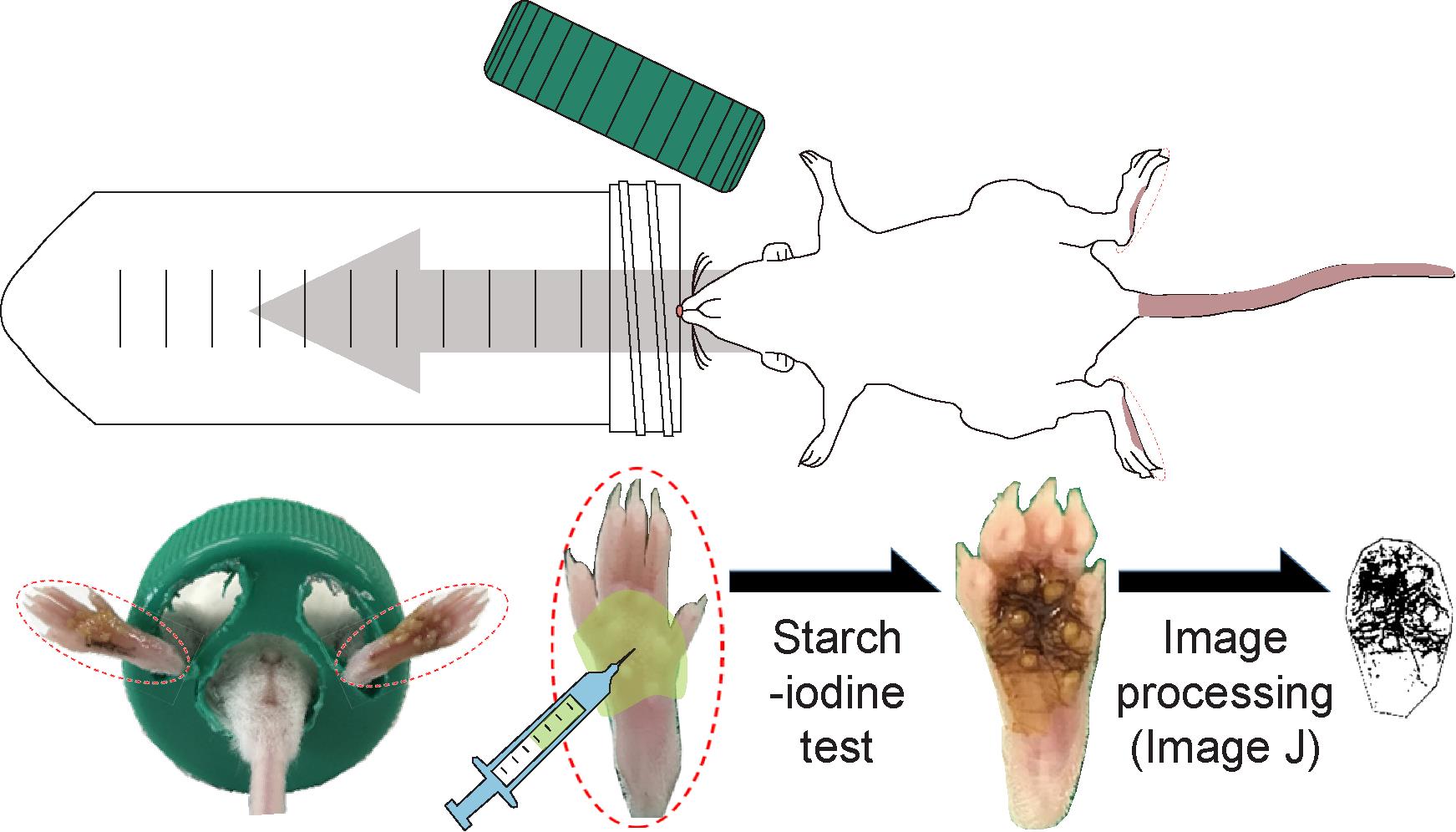
Overview of the mouse-model sweat assay and objective quantitation of the focal sweating area
Background
Numerous populations – nearly 3% of people aged 25-64 years – experience focal hyperhidrosis, an idiopathic abnormality characterized by hyper-sweating concentrated in certain regions of the body, e.g., palms, soles, face, and armpits (Haider et al., 2005). It interferes with daily activities; therefore, this condition poses substantial emotional, psychological, social, and professional burden (Strutton et al., 2004) and even leads to social anxiety disorders (Solish et al., 2007). During the development of drugs or medications for the treatment of focal hyperhidrosis, rational assays for evaluating sweating are necessary to evaluate excessive perspiration symptoms. Several tests used in clinical practice and research studies are not sensitive enough to detect subtle perspiration changes resulting from medications (Provitera et al., 2010). The thermoregulatory sweat test stimulates entire body sweating, including central and peripheral components, but does not localize the sweating lesion site (Fealey et al., 1989). Simple colorimetric procedures, such as the Neuropad test, evaluate sweating abnormalities but do not provide detailed analysis (Quattrini et al., 2008). In addition, abundant in vivo preclinical data are necessary for the verification of anti-perspiration efficacy before clinical trials; however, studies, particularly using mouse models, are rare.
The iodine-starch test is a well-known chemical reaction between starch and iodine, which instantly produces an intense blue-black color. The test was first described in 1814 (Colin et al., 1814) and first applied in 1928 as a medical test for visualizing sudomotor function (perspiration or sweating) (Minor, 1928). Polyiodides complexed with the amylose helix in starch form infinite polyiodide homopolymers and produce the blue-black color, whereas non-ionized iodine dissolved in nonpolar solvents and individual iodide (I−) do not react with starch (Madhu et al., 2016). The triiodide (I3−) ion is the simplest polyiodide, which is yellow-to-brown in aqueous conditions with no starch. In addition, the blue-black color of the triiodide-starch complex is so deep, which can also be detected visually when the concentration of iodine is as low as 2 × 10−5 M at 20°C. Consequently, the iodine-starch test has been used as a well-established diagnostic tool to evaluate underactive (hypohidrosis) and overactive (hyperhidrosis) sweating (Chia et al., 2013).
Even in the mouse sweat assays, various errors may occur owing to arbitrary judgment because the number of sweating spots, attributed to the iodine-starch reaction, is manually counted to quantitate the sweating score (Nejsum et al., 2002; Liu et al., 2017). For the data analysis, image-processing using ImageJ, a free software provided by the National Institutes of Health (NIH), can be utilized to eliminate possible arbitrary judgment and errors. In this protocol, we generated a mouse sweat-assay model and used ImageJ for image analysis (Ban et al., 2020). The sweating area on the skin of hind footpads of anesthetized mice was stained blue-black using an iodine-starch test. The blue-black pixels were extracted from the footpad images obtained, adjusted using the Auto Local Threshold function in ImageJ, and measured to the area fraction (sweating area %). Using the present protocol, a clear difference between the control and the antiperspirant-administered mice was observed with respect to the sweating area % with no error. Moreover, whether expected antiperspirants exploit either circulatory-systematic or focal anti-perspiration efficacy can also be assessed using this protocol.
Materials and Reagents
Pipet tips, 20 µl, 200 µl, and 1,000 µl (Gilson, catalog numbers: F161671, F1739311, and F161451, respectively)
0.2 μm syringe filter
Conical tube with lid, 50 ml (SPL Life Sciences, catalog number: 50050)
Microtubes, 200 µl and microtube 1.5 ml (Sigma-Aldrich, catalog number: Z374873; Eppendorf, catalog number: EP0030125150)
Sterilized needles for syringe, 17 gauge (Korea vaccine)
Sterilized syringe filter (Sartorius, catalog number: 17764-ACK)
Sterilized syringes for general use, 1 and 50 ml (Korea vaccine)
Mice (6-week-old Institute of Cancer Research, weighing 25-35 g; Koatec Co., Pyeongtaek, Korea)
Myricetin (Sigma-Aldrich, catalog number: M6760)
Glyceryl tristearate (Sigma-Aldrich, catalog number: T5016)
Glyceryl trioctanaote (Sigma-Aldrich, catalog number: T9126)
Brij S100 (Sigma-Aldrich, catalog number: 466387)
Pluronic P-123 (Sigma-Aldrich, catalog number: 435465)
Pilocarpine hydrochloride (pilocarpine) (Tokyo Chemical Industry, catalog number: P0434)
Glycopyrrolate bromide (glycopyrrolate) (Tokyo Chemical Industry, catalog number: G0392)
Neuronox 100U (botulinum neurotoxin type A, BoNT/A) (Medytox)
Alfaxalone solution, 10 mg/ml (Alfaxan) (Alfaxan Multidose, Jurox Animal Health)
Xylazine hydrochloride solution, 23.32 mg/ml (Rumpun, Bayer)
Starch (Millipore, catalog number: 101252)
Castor oil (Sigma-Aldrich, catalog number: 259853)
Iodine (Sigma-Aldrich, catalog number: 207772)
Ethanol (Sigma-Aldrich, catalog number: 02891)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855)
HyClone phosphate-buffered saline, pH 7.4 (PBS) (GE Healthcare, catalog number: SH30256.01)
Rumpun solution (see Recipes)
Anesthesia solution (see Recipes)
Pilocarpine solution (see Recipes)
Glycopyrrolate solution (see Recipes)
Myricetin solution (see Recipes)
Myricetin-loaded lipid nanoparticles (M-LNPs) (see Recipes)
Starch solution (see Recipes)
Iodine solution (see Recipes)
Equipment
Pipets (20, 200, and 1,000 µl) (Gilson, catalog numbers: FA10003M, FA10005M, and FA10006M, respectively)
Electric pharynx
Pioneer analytical balance, capacity 320 g (Ohaus, model: PX323E)
Cages for stabilizing between the time of anesthetic injection and complete anesthesia
Paintbrushes to apply iodine and starch solutions on the hind footpads of mice
Digital camera (Canon, model: EOS 800D)
Mouse restrainers were prepared by making holes in the plastic wall of conical tubes and the lid using an electric pharynx (Figure 1)
Software
ImageJ (NIH, http://rsb.info.nih.gov/ij/) for image-processing and determination of the sweating area %
SigmaPlot 10.0 Windows version (IBM Co.) for drawing graphs
SPSS Statistics version 23.0 software (IBM Co.) for the statistical analyzes
Procedure
Male mice (5 mice per cage) were housed at a temperature of 21 ± 1°C and a humidity of 55 ± 10% under controlled laboratory conditions and maintained with food and water ad libitum. Mice were tested during the light phase of a 12-h light cycle (lights on at 6:00 am and off at 6:00 pm).
Anesthesia
Six-week-old Institute of Cancer Research (ICR) mice, weighing 25-35 g, were purchased from Koatec Co. (Pyeongtaek, Korea). After arrival, the mice were housed in an animal facility for 7 days for acclimatization.
The mice were anesthetized by intraperitoneal injection of anesthesia solution (8.5 ml·kg−1; Recipe 2). All procedures for the sweat assay were terminated within 1 h before the mice woke up.
The mice were placed on a 37°C-heating pad in an empty cage for 5 min without any stimulus (e.g., noise and light) for stabilization of anesthesia.
The anesthetized mice were carefully transported into a mouse restrainer (Equipment 7) and covered using a screw-lid.
Drug administration
The drug solutions (pilocarpine, glycopyrrolate, BoNT/A, myricetin, and M-LNP, see the Recipes section) were prepared and warmed to 37°C immediately before administration. If needed, the drug solutions were sterilized by passing through a 0.2-μm syringe filter before use.
Administration of the drug solution after stabilization of anesthesia
Application on hind footpad skin (Figure 1A)
The drug solution was pipetted onto the hind footpad skin. Precautions were taken not to over-apply the solution.
A mouse-containing restrainer was placed on the heating pad for 30 min to allow sufficient time for absorption and action of the agents.
Subcutaneous injection (Figure 1B)
A needle-equipped syringe was loaded with drug solution. Care was taken not to inject the drug solution over 20 µl to avoid excessive bulging of the skin.
To remove the air in the syringe column, the piston was pressed while keeping the syringe with the needle-side up.
The needle was slowly inserted into the right hind footpad skin as close to the surface as possible, while maintaining an almost parallel angle between the needle and the skin surface to avoid subcutaneous structural damage and bleeding. Thin needles (17 gauge) were used to minimize skin damage, and fresh needles were used every time to prevent infection.
The drug solution was injected and the needle was removed. If saliva secretion stimulants such as pilocarpine hydrochloride were used, saliva overflow was eliminated using a wipe to prevent suffocation.
Any overflow was wiped off.
The mouse-containing restrainer was placed down on the heating pad for 10 min to allow sufficient time for action of the agents.
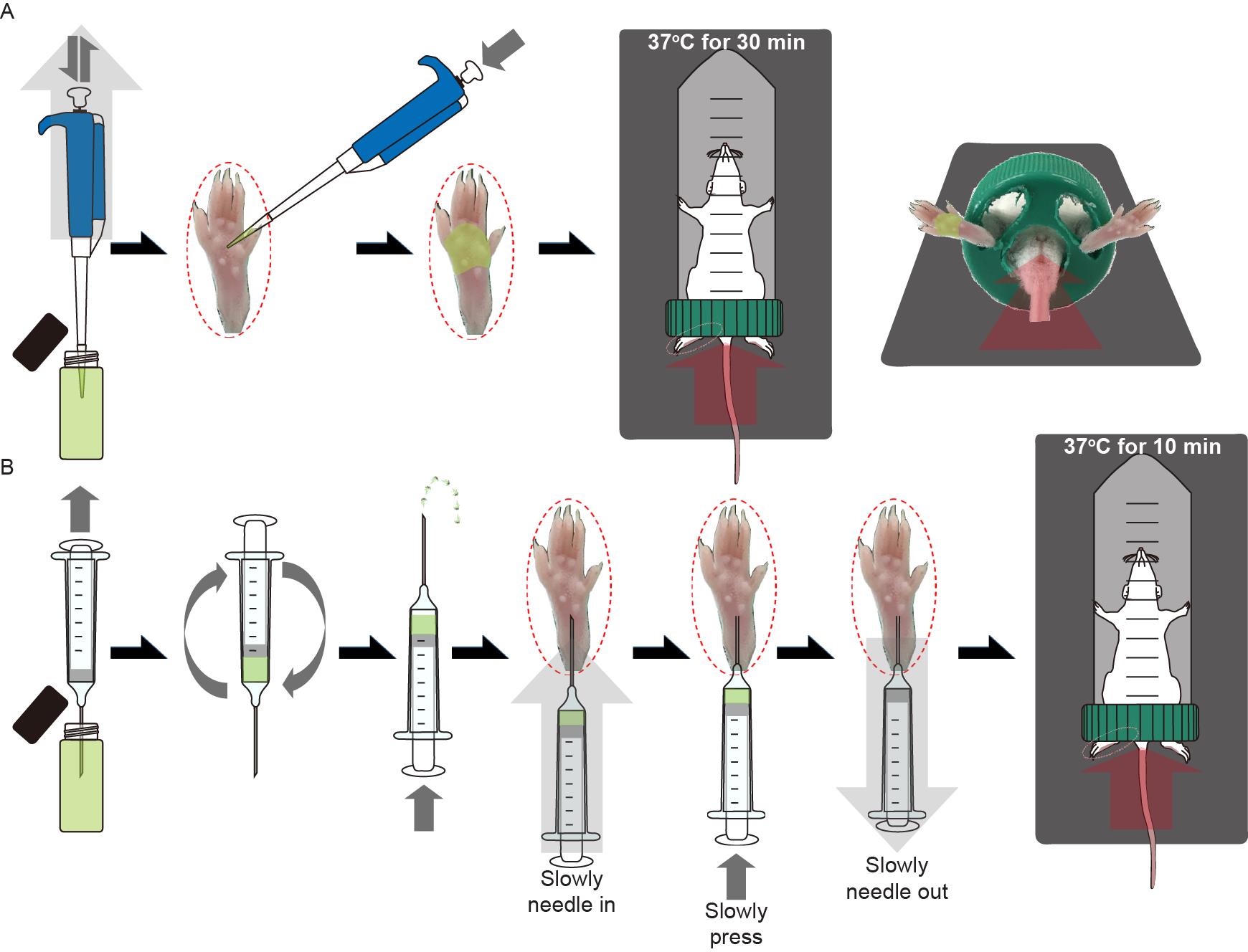
Figure 1. Administration of the drugs in the sweat assay after anesthesia. A. Procedures for the application of the drug solutions on the skin of the right hind footpads of mice. B. Procedures for the subcutaneous injection of the drug solutions into the right hind footpads of mice.Iodine-starch test (Figure 2)
After administration of the drugs (pilocarpine, glycopyrrolate, BoNT/A, myricetin, and M-LNP), the remainder of the solution was wiped off the footpad skin, the skin washed with warm PBS, and the liquid wiped off again.
Using a paintbrush, the iodine solution was applied to both the left and right hind footpads and allowed to dry for 1 min. The paintbrush used was wider than the horizontal width of the hind footpads, which enabled painting of enough iodine solution at once. Further, the paintbrush was soaked in iodine solution before use to avoid the formation of iodine crystals resulting from ethanol-drying. To avoid the formation of spots with iodine crystals, which can influence the values of the resulting sweating area %, application was done only once.
To block sweat evaporation from the skin surface, a coat of starch-oil solution was applied once on the footpad skin by brushing at 37°C. A different paintbrush was used for this step. To avoid starch aggregation during the application, the starch solution was stirred.
Immediately after coating with the starch solution, photographs of the hind footpad were acquired under fixed conditions of distance, magnification, and exposure. Atmospheric exposure of the starch-covered hind footpads for longer than 5 min can affect the unwanted color change due to moisture; thus, we suggest 5 min as the time limit for determining the sweating area (%).
Description of the test: The iodine-in-ethanol solution was first applied over the footpad skin and the ethanol was then evaporated for 1 min. Next, the starch-in-oil solution was applied over the footpad skin covered with iodine. The sweat is captured between the skin and the oil layer since the moisture cannot diffuse out through the hydrophobic oil layer. In turn, dissolution into the captured sweat makes the brownish iodine react with the starch and turns a blue-black color as a result of ionization to polyiodide ions. In addition, the concentrations of starch and iodine in the starch solution and iodine solution, respectively, are high enough to induce the blue-black color change if the mice sweat.
Note: The number of solution applications, once each, is more important than the volume. Therefore, the color can be changed to blue-black by each serial application of the iodine and starch solutions only once, without any risk of a lack of color change.
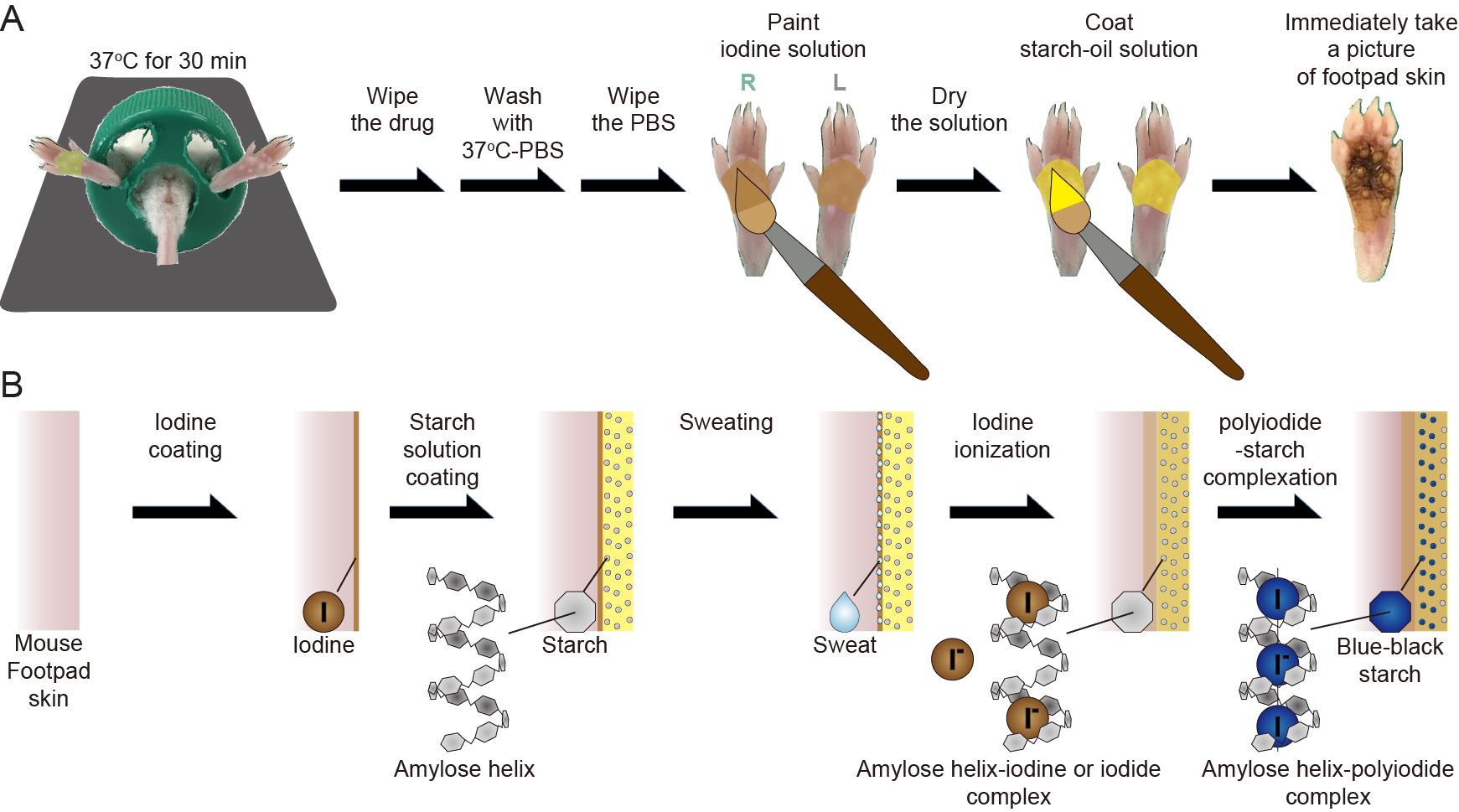
Figure 2. Iodine-starch test in the sweat assay. A. Procedures of the test. B. Illustration of an expected mechanism for the test.
Data analysis
Image-processing and sweating area % determination
Area selection (Figure 3A): The images of the hind footpad skin of mice were individually processed in ImageJ and only the footpad area was cropped.
Modifying the images (Figure 3A): “Image > Type > RGB stack”, the cropped real color images of the footpad skin area were converted into grayscale.
Auto local threshold (Figure 3B): The grayscale images of the blue stack were adjusted by selecting “Image > Adjust > Auto Local Threshold” using the Phansalkar method. Radius: 15; Parameter 1 and 2: 0; check “White objects on black background”; uncheck “Stack.” Black pixels in the adjusted images indicate the sweating spot on the hind footpad skin. As shown in Figure 3C, the Phansalkar method is the best way to select the sweating spots among all the modules available the Auto Local Threshold.
Note: Do not touch any initial settings for the Auto Local Threshold except the Phansalkar method selection.
Determination of the sweating area % (Figure 3D): The measurements were set by selecting “Analyze > Set Measurements” and checking “‘Area fraction” before the measurement. The area fraction values of the black pixels in the adjusted images were measured and recorded by either pressing the “Ctrl + M” keys on a keyboard or clicking “Analyze > Measure.” The sweating area % was calculated by subtracting the area fraction from 100%.
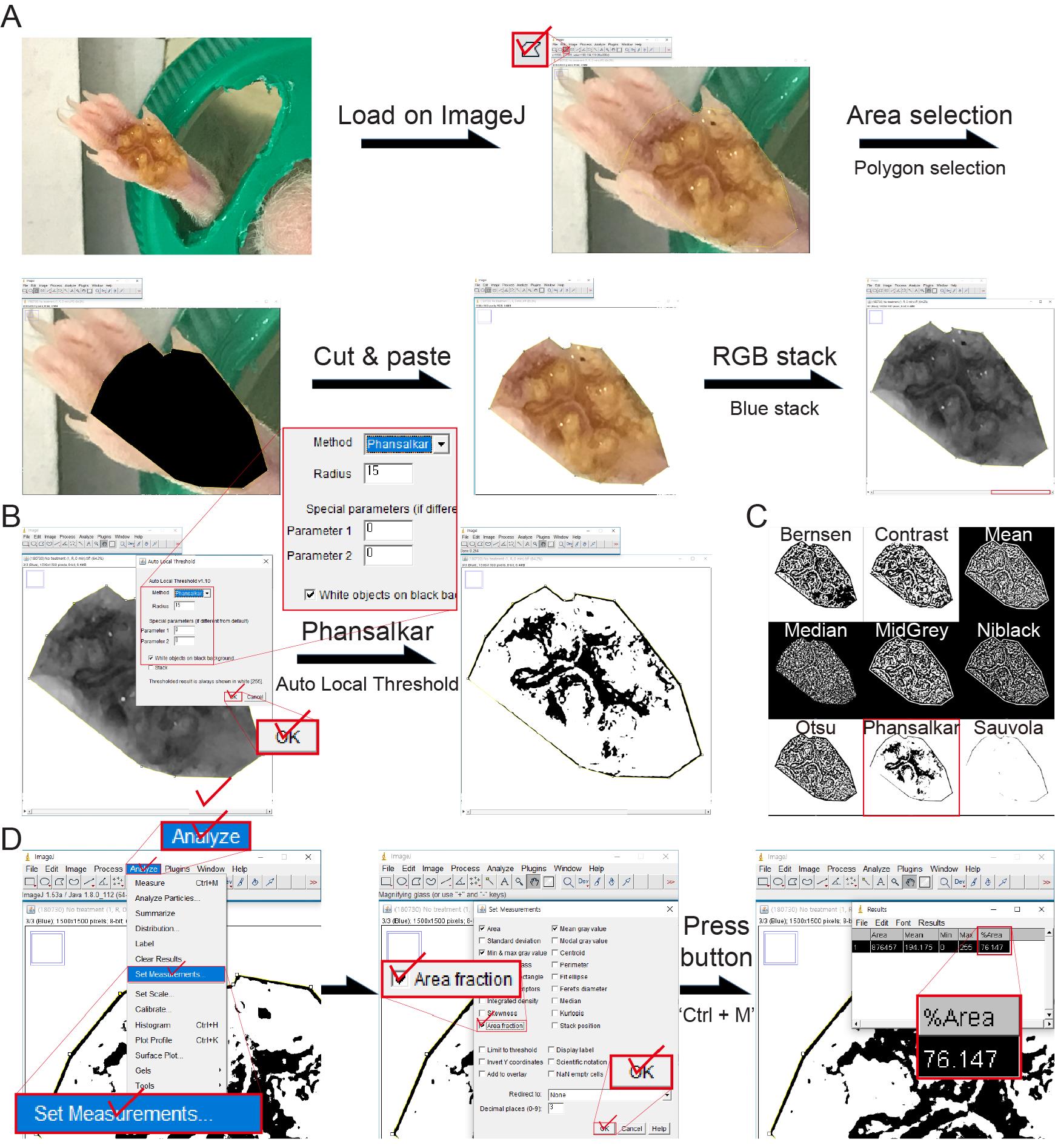
Figure 3. ImageJ-based image-processing and measurement of the sweating area %. A. Area-selection of the mouse footpad skin in the obtained image and conversion of the selected image into grayscale (the blue stack among red, green, and blue stacks) using RGB stack. B. Adjustment of the blue stack-based grayscale image using the Auto Local Threshold, according to the Phansalkar method, for extracting the sweating area. C. The adjusted images using all the modules for the Auto Local Threshold, including the Bernsen, Contrast, Mean, Median, MidGrey, Niblack, Otsu, Phansalkar, and Sauvola methods. D. Establishing the method for the measurement of the Area fraction that is used for determining the sweating area % values.Assessment of the reliability of image-processing
All the sweating area % values of each group were determined from the footpad skin images obtained at different predetermined times (0, 1, 2, 3, 4, and 5 min) after the iodine-starch test using ImageJ (Figure 4A and 4B). The reliability of the image-processing for determining the sweating area % values of each group was assessed using the coefficient of determination (R2) of the linear-fitting curves, as shown in Figure 4C. Reliable data: R2 ≥ 0.9; unreliable data: R2 < 0.9. Unreliable datasets were excluded when determining the sweating area % values of a certain group.
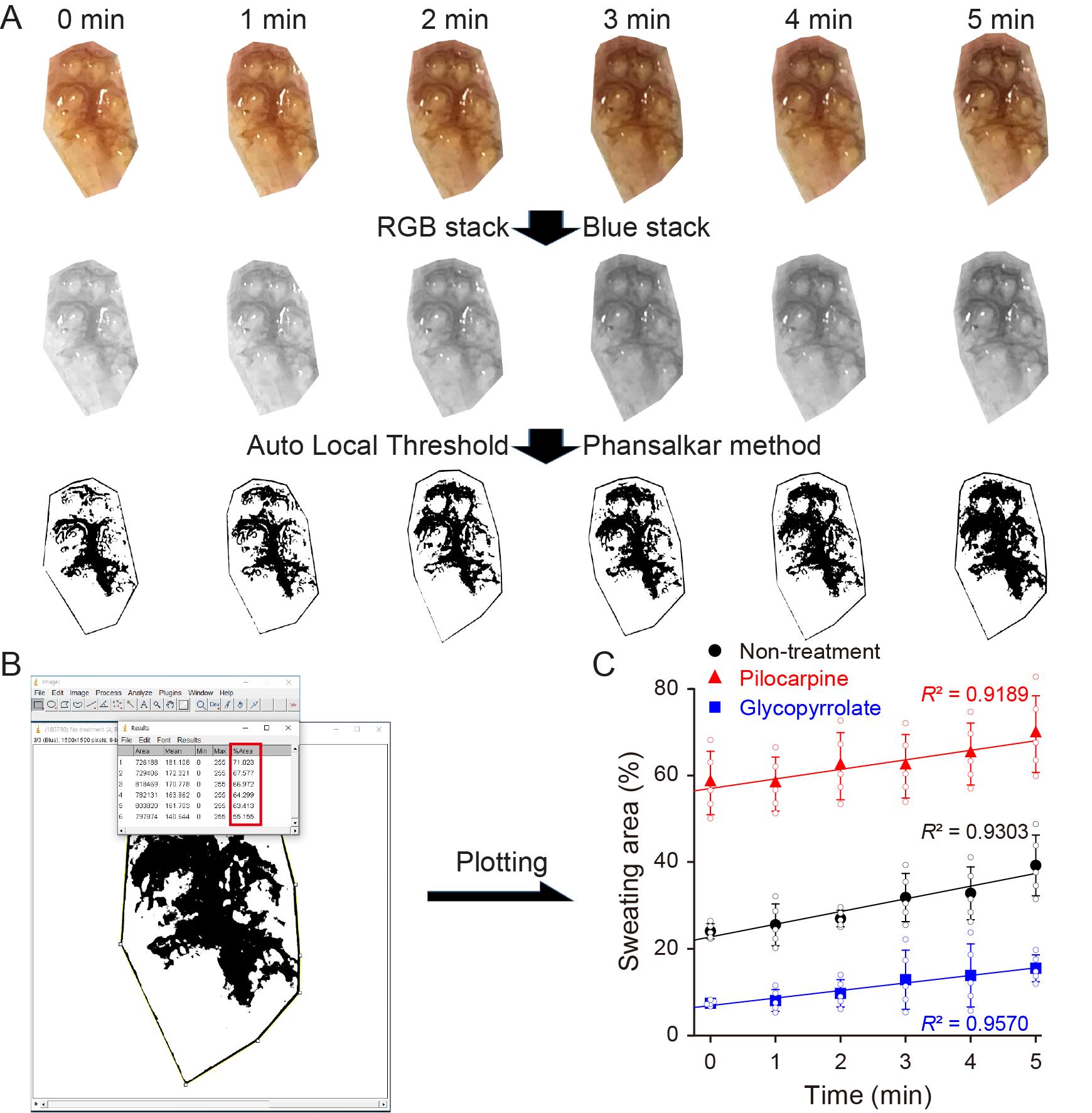
Figure 4. Reliability assessment of the image-processing procedures for determining the sweating area %. A. Procedures for the representative image-processing of the right hind footpad skin that is not administered with any drug (non-treatment) for 5 min after the initiation of the iodine-starch test. B. Area fraction values (%Area) determined 0, 1, 2, 3, 4, and 5 min after the procedures for the representative image-processing. C. The linear-fitting curves for the sweating area % values of non-treatment, negative control (Pilocarpine) and positive control (Glycopyrrolate) groups. Data are expressed as the mean ± SD (standard deviation; n = 5).Statistical analysis and plotting the graph for the sweating area %
All data from the sweat assay are reported as the average and standard deviation (SD) of at least five independent mouse experiments. Statistical analyses were performed using a Student’s t-test with SPSS Statistics. The bar graph including the average and SD of the sweating area % value for each mouse group was generated using SigmaPlot 10.0 (Figure 5).
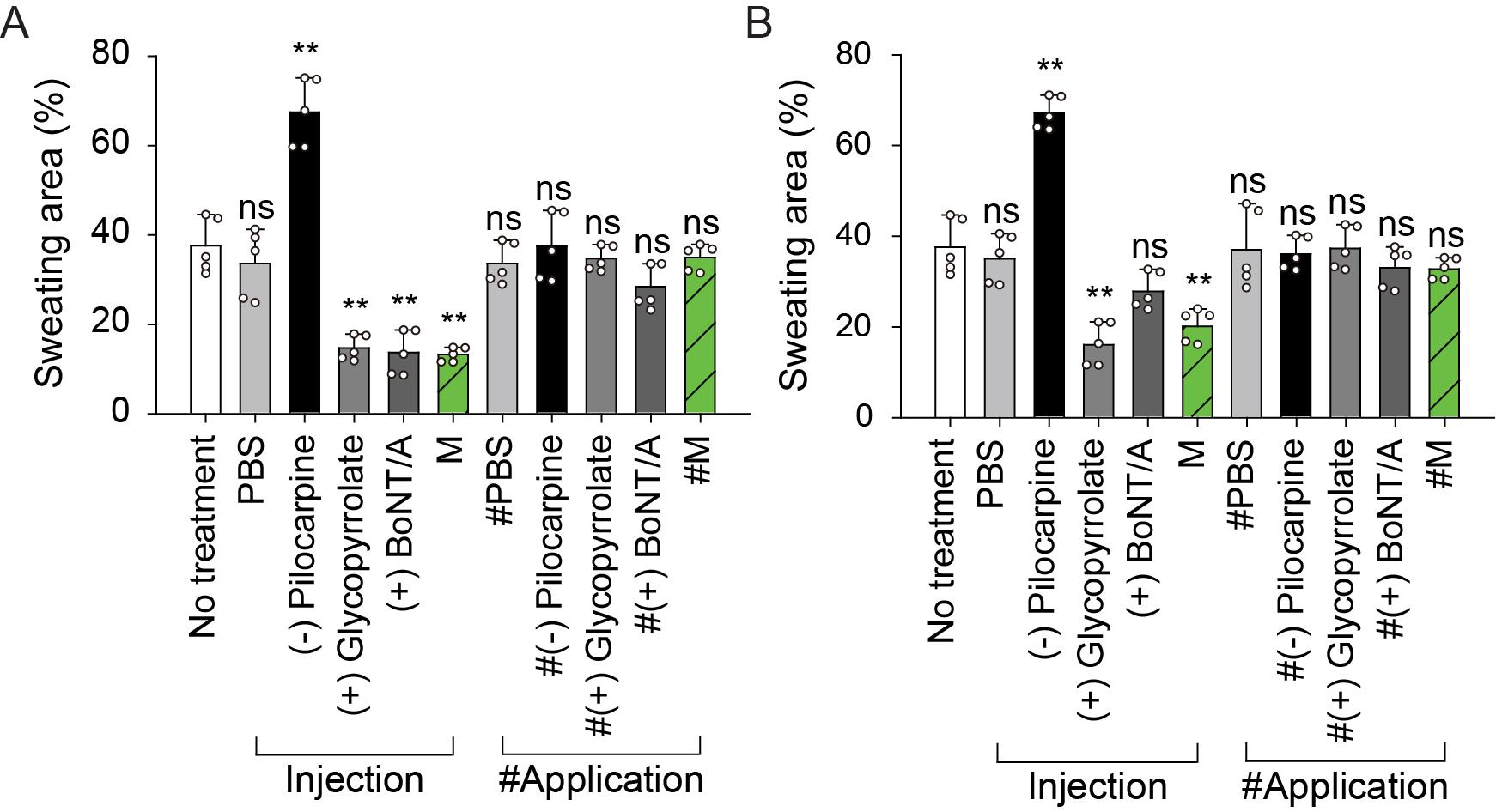
Figure 5. Representative sweating area % values of the mouse hind-footpad skin 5 min after the iodine-starch test. Sweating area % values (%Area of black pixel fraction) on (A) right hind footpads, directly treated (by either subcutaneous injection or applied on the skin), and (B) left hind footpads, not treated with drug. Groups: no treatment, PBS (0.8 ml/kg), pilocarpine HCl (2.5 mg/kg, negative control), glycopyrrolate bromide (0.25 mg/kg, positive control), BoNT/A (0.8 U/kg, positive control), myricetin (M; 0.8 mg/kg), and M-LNP (myricetin-loaded lipid nanoparticle; 0.8 ml/kg). Data are expressed as the mean ± SD (n = 5; Student’s t-test; ns, non-significant; *, P < 0.05; **, P < 0.01).Assessment of the focal anti-perspiratory effect of drugs
Sweating area % values for left hind footpads, to which drugs were not administered, are represented as separate graphs to assess the circulatory-system or topical efficacy of the drugs. Herein, the sweating area % reduction in both the right and left footpads indicates the circulatory-system anti-perspiration efficacy of the drug, whereas that only in the right footpad indicates the topical efficacy.
Recipes
Rumpun solution
Rumpun (xylazine hydrochloride, 23.32 mg/ml) diluted with PBS to 20 mg/ml xylazine hydrochloride
Anesthesia solution
Mixture of Alfaxan and Rumpun solution at a volumetric ratio of 16:1
Pilocarpine solution
Pilocarpine hydrochloride diluted with PBS to 3.125 mg/ml
Glycopyrrolate solution
Glycopyrrolate bromide diluted with PBS to 312.5 μg/ml
Myricetin solution
5% (w/v) myricetin solution (in DMSO) diluted with PBS to 1 mg/ml immediately before administration to prevent crystal formation
Myricetin-loaded lipid nanoparticles (M-LNPs)
M-LNPs prepared using the method previously reported by our group (Ban et al., 2020); concentration of myricetin in the M-LNP system: 1 mg/ml
Starch solution
10% (w/v) starch dispersed in castor oil and vortexed immediately before use to prevent sedimentation
Iodine solution
3.5% (w/v) iodine dissolved in ethanol
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C2101964, 2017R1A6A1A03015642, and 2018R1D1A1B07050508). This protocol was derived from Ban et al. (2020).
Competing interests
There are no competing interests to be declared.
Ethics
This study was approved by the Ethics Committee of Sungkyunkwan University (approval no. SKKUIACUC2018-04-11-2). All mice included in this study were housed in a facility at Sungkyunkwan University, and all experimental protocols were approved by the Animal Care and Use Committee of Sungkyunkwan University.
References
- Ban, C., Park, J. B., Cho, S., Kim, H. R., Kim, Y. J., Choi, Y. J., Chung, W. J. and Kweon, D. H. (2020). Reduction of focal sweating by lipid nanoparticle-delivered myricetin. Sci Rep 10(1): 13132.
- Chia, K. Y. and Tey, H. L. (2013). Approach to hypohidrosis. J Eur Acad Dermatol Venereol 27(7): 799-804.
- Colin, J. J. and Gaultier de Claubry, H. F. (1814). Mémoire sur les combinassions de l'iode avec les substances végétales et animaux. Ann Chim Phys 90: 87-110.
- Fealey, R. D., Low, P. A. and Thomas, J. E. (1989). Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc 64(6): 617-628.
- Haider, A. and Solish, N. (2005). Focal hyperhidrosis: diagnosis and management. CMAJ 172(1): 69-75.
- Liu, Y., Sebastian, B., Liu, B., Zhang, Y., Fissel, J. A., Pan, B., Polydefkis, M. and Farah, M. H. (2017). Sensory and autonomic function and structure in footpads of a diabetic mouse model. Sci Rep 7: 41401.
- Madhu, S., Evans, H. A., Doan-Nguyen, V. V., Labram, J. G., Wu, G., Chabinyc, M. L., Seshadri, R. and Wudl, F. (2016). Infinite Polyiodide Chains in the Pyrroloperylene-Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes. Angew Chem Int Ed Engl 55(28): 8032-8035.
- Minor, V. (1928). Ein neues Verfahren zu der klinischen Untersuchung der Schweißabsonderung. Dtsch Z Nervenheilkd 101(1): 302-308.
- Nejsum, L. N., Kwon, T. H., Jensen, U. B., Fumagalli, O., Frokiaer, J., Krane, C. M., Menon, A. G., King, L. S., Agre, P. C. and Nielsen, S. (2002). Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A 99(1): 511-516.
- Provitera, V., Nolano, M., Caporaso, G., Stancanelli, A., Santoro, L. and Kennedy, W. R. (2010). Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 74(1): 50-56.
- Quattrini, C., Jeziorska, M., Tavakoli, M., Begum, P., Boulton, A. J. and Malik, R. A. (2008). The Neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia 51(6): 1046-1050.
- Solish, N., Bertucci, V., Dansereau, A., Hong, H. C., Lynde, C., Lupin, M., Smith, K. C., Storwick, G. and Canadian Hyperhidrosis Advisory, C. (2007). A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg 33(8): 908-923.
- Strutton, D. R., Kowalski, J. W., Glaser, D. A. and Stang, P. E. (2004). US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 51(2): 241-248.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ban, C. and Kweon, D. (2021). Objective Quantitation of Focal Sweating Areas Using a Mouse Sweat-assay Model. Bio-protocol 11(11): e4047. DOI: 10.21769/BioProtoc.4047.
Category
Neuroscience > Sensory and motor systems > Animal model
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








