- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
ODELAM: Rapid Sequence-independent Detection of Drug Resistance in Mycobacterium tuberculosis Isolates
(*contributed equally to this work) Published: Vol 11, Iss 10, May 20, 2021 DOI: 10.21769/BioProtoc.4027 Views: 4731
Reviewed by: Emilia KrypotouRon Saar DoverAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

SIMBA Method—Simultaneous Detection of Antimicrobial and Anti-biofilm Activity of New Compounds Using Salmonella Infantis
Meta Sterniša [...] Anja Klančnik
Aug 5, 2023 2009 Views

Functional Assay for Measuring Bacterial Degradation of Gemcitabine Chemotherapy
Serkan Sayin and Amir Mitchell
Sep 5, 2023 1741 Views

Identification of Mycobacterium tuberculosis and its Drug Resistance by Targeted Nanopore Sequencing Technology
Chen Tang [...] Guangxin Xiang
Feb 5, 2025 1985 Views
Abstract
Antimicrobial-resistant Mycobacterium tuberculosis (Mtb) causes over 200,000 deaths globally each year. Current assays of antimicrobial resistance require knowledge of the mutations that confer drug resistance or long periods of culture time to test growth under drug pressure. We present ODELAM (One-cell Doubling Evaluation of Living Arrays of Mycobacterium), a time-lapse microscopy-based method that observes individual cells growing into microcolonies. This protocol describes sample and media preparation and contains instructions for assembling the ODELAM sample chamber. The ODELAM sample chamber is designed to provide a controlled environment to safely observe the growth of Mtb by time-lapse microscopy on an inverted wide-field microscope. A brief description of the ODELAM software is also provided here. ODELAM tracks up to 1500 colony forming units per region of interest and can observe up to 96 regions for up to seven days in a single experiment. This technique allows the quantification of population heterogeneity. ODELAM enables rapid quantitative measurements of growth kinetics in as few as 30 h under a wide variety of environmental conditions.
Graphic abstract:
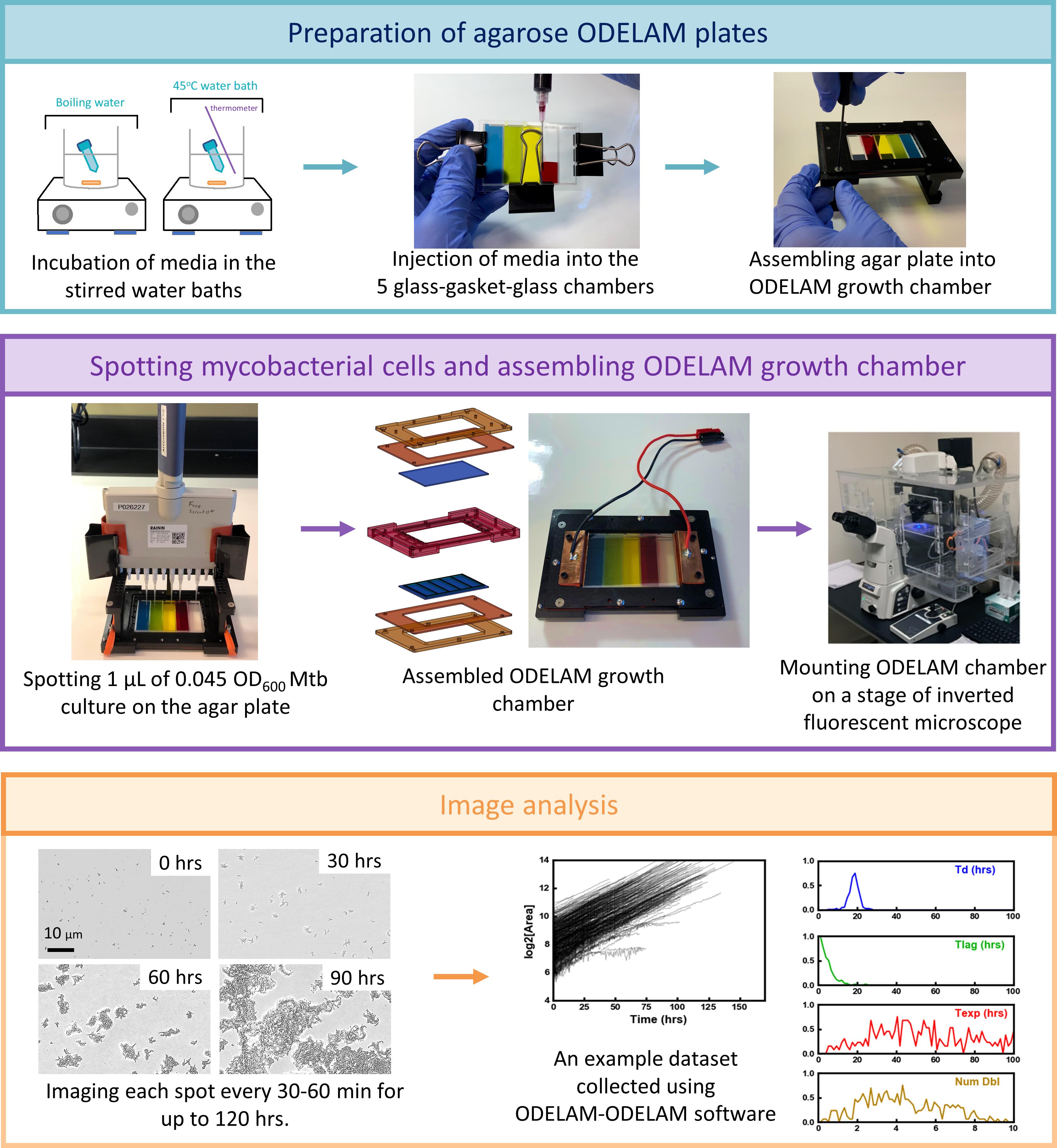
Schematic representation of the ODELAM platform
Background
Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis (TB), which is responsible for 1.4 million deaths globally each year (World Health Organization, 2020). Despite decades of research, TB treatment is still challenging, and current chemotherapies require the combination of four different drugs (Pai et al., 2016). One of the reasons why Mtb remains a remarkably successful pathogen is its ability to produce heterogeneous phenotypes that exhibit variable responses to a given drug (Manina et al., 2015; Dhar et al., 2016; Rego et al., 2017; Logsdon et al., 2018). Thus, characterizing the growth phenotypes of individual Mtb cell populations is critical for understanding how mycobacteria sense and respond to stress conditions such as prolonged drug exposure (Santi et al., 2013; Logsdon et al., 2018).
To date, little is known about the sources and consequences of cell-to-cell differences, mostly due to limitations in the tools and techniques available for precise measurement of growth phenotypes of individual bacterial cell populations. Mtb is especially challenging to study due to its very long doubling time of approximately 24 h. Recent platforms to study single bacterial cells are limited either by the number of cells that can be observed or by the extensive time required (few weeks) to monitor the growth of mycobacterial colonies (Aldridge et al., 2012; Golchin et al., 2012; Wakamoto et al., 2013; Choi et al., 2014; Manina et al., 2015; Barr et al., 2016).
ODELAM (One-cell Doubling Evaluation of Living Arrays of Mycobacterium) was developed to overcome these limitations and is based on ODELAY, developed originally to analyze yeast (Herricks et al., 2017a, 2017b and 2020). ODELAY is a versatile method that has been utilized to investigate the structure-function relationship of the nuclear pore complex and peroxisome biogenesis (Kim et al., 2018; Mast et al., 2018). ODELAM uses time-lapse microscopy to quantify growth phenotypes of populations of individual Mtb colony-forming units (CFU). By direct monitoring of bacterial growth, we can assess the heterogeneity of Mtb strains, arising either naturally or in response to stresses, such as drug treatment. ODELAM can observe up to 1500 CFU per region of interest (ROI) and measure four main growth kinetic parameters: lag time, doubling time, exponential time (the time when colonies stop growing and enter stationary phase), and number of doublings for each identified CFU. ODELAM can track up to 100,000 CFUs in one experiment, which provides statistical power to detect phenotypic variations in the population. Importantly, ODELAM can screen between 80 and 96 samples growing in up to five media conditions within each experiment. In less than 48 h, ODELAM identifies resistant cells in a population of sensitive bacteria down to 1 per 1000 and predicts the minimum inhibitory concentration (MIC) of drugs with high accuracy. Additionally, the ODELAM platform is broadly applicable as a laboratory screening tool, as it can be easily adapted to study most colony-forming microorganisms. ODELAM can quantify heterogeneity and detect heteroresistance in Mtb clinical isolates. This cutting-edge technology will have a meaningful impact on studies on bacterial phenotypic heterogeneity and emergence of drug resistance and help to better understand bacterial adaptation to unfavorable environmental conditions, which will facilitate the development of new powerful therapies.
Materials and Reagents
Mycobacterium tuberculosis culture
1.8 ml cryotubes (Nunc, catalog number: 375418)
50 ml conical bottom tubes (Sarstedt, catalog number: 62.547.254)
10 ml serological pipettes (VWR, catalog number: 89130-898)
200 μl tips (Genesee Scientific, catalog number: 24-412)
1,000 μl tips (Genesee Scientific, catalog number: 24-430)
Steriflip-GP Sterile Centrifuge Tube Top Filter Unit (MilliporeSigma, catalog number: SCGP00525)
Mtb strains (storage: -80°C)
H37Rv (background strain available from BEI, catalog number: NR-123)
Other strains of interest
Difco Middlebrook 7H9 Broth (BD, catalog number: 271310, storage: room temperature (RT))
Middlebrook OADC Enrichment (BD, catalog number: 212351, storage: 4°C)
Glycerol (Sigma Aldrich, catalog number: G5516, storage: RT)
Tween 80 (Sigma-Aldrich, catalog number: P1754, storage: RT protected from light)
Disinfectants:
70% ethanol (Fisher Scientific, catalog number: 04-255-92, storage: RT)
PREempt RTU Disinfectant Solution (Contec, catalog number: 21105, storage: RT)
LoPhene Concentrate Rotational Disinfectant (Decon Labs, catalog number: 8801, storage: RT)
ODELAM agarose gel preparation
50 ml conical bottom tubes (Falcon, catalog number: 352070)
15 ml conical bottom tubes (Falcon, catalog number: 352096)
5 ml syringe (BD, catalog number: 309646)
18 G × 1½ needle (BD, catalog number: 305196)
Micro Slides 2 in × 3 in 1 mm thick (VWR, catalog number: 48382-180)
UltraPure agarose (Invitrogen, catalog number: 16500, storage: RT)
Difco Middlebrook 7H9 Broth (BD, catalog number: 271310, storage: RT)
Middlebrook OADC Enrichment (BD, catalog number: 212351, storage: 4°C)
Glycerol (Sigma Aldrich, catalog number: G5516, storage: RT)
ODELAM spotting cell cultures
96-well plate, F-bottom (Greiner bio-one, catalog number: 655180)
Pipette tips RT LTS 20µL 960A/10 (Raining, catalog number: 30389200)
7H9-GOT media (see Recipes)
10× 7H9-G media (see Recipes)
Equipment
BSL1 or BSL2
Pipettes
Rainin 2-20 μl, 20-200 μl, 100-1,000 μl
0.1-10 μl 12-multichannel pipette (Pipet-Lite Multi Pipette L12-10XLS+) (Rainin, catalog number: 17013807)
Note: The ODELAM chamber is designed for this specific pipette.
2 × hot plate stirrer (Corning, catalog number: PC-420D)
2 × 1 L beakers (Sigma-Aldrich, catalog number: CLS10001L-1EA)
2 × stir bars (VWR, catalog number: 58948-150)
2 × glass giant Petri dishes to cover the beakers
Vortexer (Scientific Instruments, model: Vortex-Genie 2, catalog number: SI-0256)
Thermometer (VWR, catalog number: 89095-640)
Top-loading Electronic scale with 1 kg capacity and 0.01 g accuracy
Medium (3 per one 5 glass-gasket-glass chambers) and large (2 per one single glass-gasket-glass chamber) binder clips
3D printer (e.g., Ender 3D, BCN3D Sigma v19, makerbot 2 ×)
BSL3
Pipettes:
Eppendorf 2-20 μl, 20-200 μl, 100-1,000 μl
0.1-10 μl 12-multichannel pipette (Pipet-Lite Multi Pipette L12-10XLS+) (Rainin, catalog number: 17013807)) with attached binder clips
Motorized inverted microscope with heated incubator (tested on Leica DMI6000 and Nikon TiE)
Personal Protective Equipment (PPE):
Double nitrile gloves
Protective Tyvek coveralls
Shoe covers
Powered air-purifying respirator (PAPR)
Absorbent pad (for working in the biosafety cabinet)
ODELAY microscope chamber (Figure 1)
Top Plate (machined from 6061-T6 aluminum)
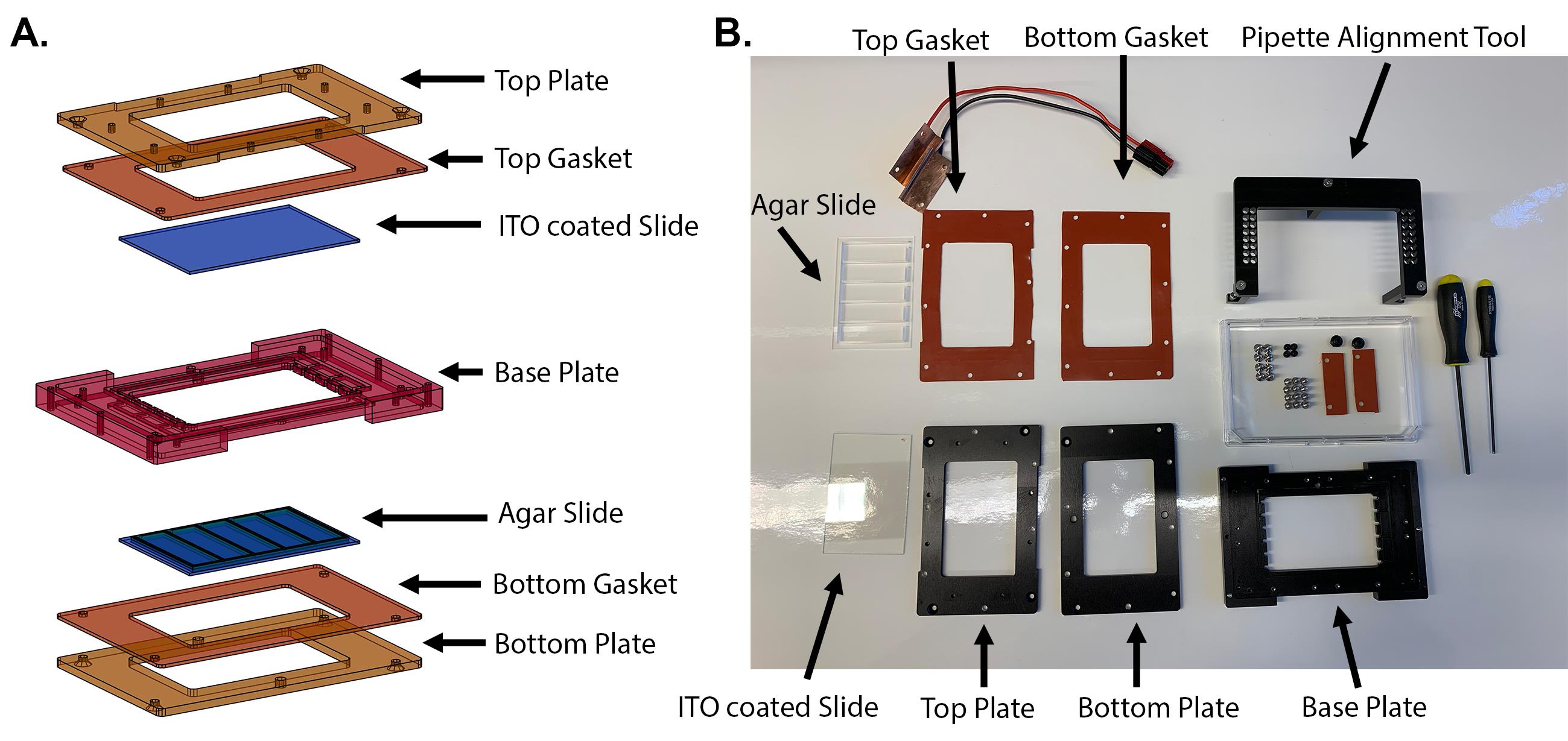
Figure 1. ODELAM sample chamber. The sample chamber for ODELAM experiments is shown in an exploded view (A) and disassembled with all relevant parts (B).Top PDMS Gasket (cut from McMaster-Carr Durometer 30A 9010K11)
Indium Tin Oxide (ITO) coated -glass slide 1.1 mm thick (SPI Supplies, catalog number: 06404-AF)
Base Plate (machined from 6061-T6 aluminum)
Glass slide with 3D printed gasket and cast agarose media
Bottom PDMS gasket (cut from McMaster-Carr Durometer 30A 9010K11)
Bottom plate (machined from 6061-T6 aluminum)
Pipette spotting alignment tool (machined from 6061-T6 aluminum)
1/16 hex screwdriver (McMaster-Carr, catalog number: 5497A23)
1/16 hex screwdriver (McMaster-Carr, catalog number: 5497A23)
12 × 4-40 5/8 inch stainless steel button-head screws (McMaster-Carr, catalog number: 98164A433)
8 × 4-40 1/4 inch stainless steel flat-head screws (McMaster-Carr, catalog number: 90585A200)
4 × 4-40 1/8 inch nylon socket head screws (McMaster-Carr, catalog number: 95868A254)
Copper electrical contacts (cut from a copper sheet)
37 °C incubator (VWR 1545 Digital Incubator)
Culture rotator (GEL GRO Tissue culture rotator, LAB-LINE)
Software
Anaconda Python (https://www.anaconda.com/)
Visual Studio Code (https://code.visualstudio.com/)
ODELAY software package (https://github.com/AitchisonLab/)
MicroManager 1.4 or 2.0 gamma (https://micro-manager.org/)
Microsoft Office Excel or another spreadsheet-compatible program (https://www.office.com/)
Cura 3D modeling (https://ultimaker.com/)
Procedure
Mycobacterium tuberculosis culture
Note: All work with Mtb should be conducted in a Biosafety Level 3 (BSL3) laboratory by trained personnel wearing appropriate PPE and be approved by the Institutional Biosafety Committee (IBC) and Environmental Health and Safety (EHS).
Prepare the pre-culture by inoculating 10 ml of 7H9-GOT (Middlebrook 7H9 media supplemented with 0.2% glycerol, 10% OADC supplement, and 0.05% (v/v) Tween-80) with 0.5 ml of the thawed Mtb glycerol stock (1:20 dilution). Grow cells in 50 ml conical tubes at 37°C for 48-72 h, until cultures reach an OD600 of 0.2-0.4 (early logarithmic phase).
Subculture by diluting 0.5 ml of the pre-culture in 10 ml of 7H9+GOT (1:20 dilution). Grow cells in 50 ml conical tubes at 37°C for 48-72 h, until cultures reach an OD600 of 0.2-0.4 (early logarithmic phase).
Dilute the culture for spotting on the ODELAM plate (see Part E of Procedure for further instructions).
ODELAM agarose aliquot
Gather the 1 L media bottle, high purity agarose, pipettes, and Falcon tubes.
Weigh 2 ± 0.02 g of agarose and place into 1 L media bottle.
Add 150 g of 18 MΩ H2O to the media bottle.
Record the total weight of the bottle, agarose, and water.
Microwave the media bottle, water, and agarose until all agarose is dissolved.
Weigh the media bottle, water, and agarose again to evaluate the amount of water lost during boiling.
Add 18 MΩ H2O until the mass equals the previously recorded total weight.
Aliquot agarose by mass, 15.1 g into 50 ml Falcon conical tubes or 3.0 g into a 15 ml Falcon conical tube.
ODELAM agarose media preparation
Note: The steps in this section can be performed outside of a biological safety cabinet if the lab is relatively dust free.
Clean the previously printed gasket slide (see 3D printing gaskets of Notes section) using lab labeling tape.
Heat two 1 L beakers with approximately 750 ml of Di H2O, one with boiling water and the other with water warmed to 40°C.
Add the appropriate amounts of 10× media and additional reagents needed to the prepared agarose aliquots (Table 1).
Note: Do not add reagents that are temperature sensitive.
Table 1. Volume recipes for the 7H9-GO Media
Final Media Volume 1.33% w.v. agarose 10× 7H9-G media 18 MΩ H2O OADC 1,000× Drug (optional)
20 ml 15.1 g 2 ml 1 ml 2 ml 20 μl 10 ml 7.5 g 1 ml 0.5 ml 1 ml 10 μl 4 ml 3.05 g 0.4 ml 0.2 ml 0.4 ml 4 μl
Clean a 50 mm × 75 mm × 1 mm glass slide using 70% ethanol. When dry, wipe with lens paper to remove any residual particles and fibers.
Place the clean glass slide on top of the printed gasket slide and secure slides with binder clips (Figure 2A).
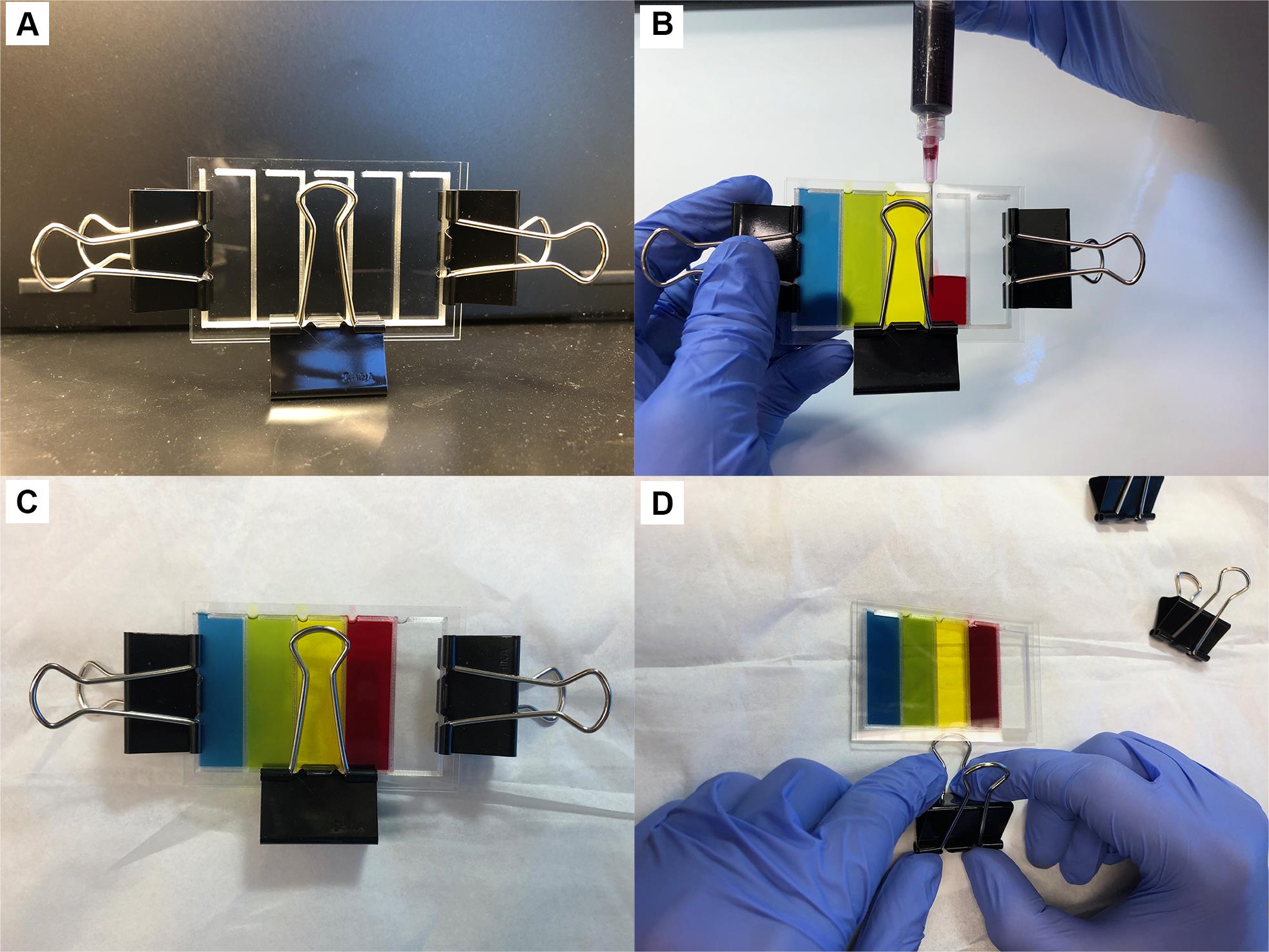
Figure 2. Assembly of agar slide. A. Ensure the gaskets are clamped directly over the gasket; otherwise, the glass will flex, and the agar will be uneven. B. Fill the slides with agarose media by injecting with a syringe needle. C-D. After allowing the media to set, use binder clip loops to pry slides apart gently (D).Assemble the syringes and 20-gauge needles for each media condition.
Place the tube with the media formulation in boiling water. The 20 ml media tubes should be in the boiling water for 18 min and the 4 ml media tubes for 8 min.
Note: Make sure the cap does not go below the water surface as this will cause water to leak into the tube. Additionally, ensure the beaker is covered; otherwise, the agarose will not melt completely.
After boiling the appropriate amount of time and the agarose is melted, vortex the agarose media tube and place it in the 40°C water bath.
Wait 3-5 min for the agarose to equilibrate at 40°C and then add the temperature-sensitive components (e.g., drug and OADC additive).
Vortex the tubes thoroughly to mix the media and additives.
Quickly draw the media into the syringe and then push the plunger slightly down and back up to remove air bubbles from the syringe.
Fill the glass slide by injecting the media into the gasket slide assembly (Figure 2B). Note which media is injected into each chamber.
Wait approximately 20-30 min for the agarose to solidify (Figure 2C).
Remove the binder clips.
Using a binder clip loop (or other convenient leverage), slowly and carefully pry the glass slides apart (Figure 2D). Try to keep the agarose from sticking to the blank slide.
Note: This step takes some practice.
Store the agarose slide in a clean plastic container (e.g., an empty tip box) for transport into the BSL-3 environment. Agarose slides can be stored 3-4 h at RT. Only use agarose slides prepared on the day of the ODELAM experiment.
Preparation for ODELAM experiment
Select the cultures and media conditions of interest.
Prepare directories and data storage space. Create file directories and ensure there is sufficient storage capacity for the experiment.
Create-Spot layout files. Double-check the file to ensure the strain layout is correct. Common mistakes include not correcting for flipping the array and not sorting the array correctly.
ODELAM Spotting Cell Cultures
Clean and sterilize all ODELAM chamber components (except the agarose slide and electrical contacts) with 70% ethanol and dry them with Kimwipes®.
Use a clean plastic container to transfer the ODELAM chamber components to the Biological Safety Cabinet.
Measure the OD600 of all Mtb cultures.
Dilute cultures to an optimal OD600. The goal is to have 500-1,000 cells per ROI. The ratio of OD600 to CFU varies from strain to strain; therefore, the OD600 value may need to be adjusted slightly for different organisms and strains. An OD600 value of 0.03-0.05 is optimal for many mycobacterium strains.
Array 150 μl of the diluted cultures into a 96-well plate. The locations in the 96-well plate should be determined by the spot layout pattern (see examples in Figures 5 and 6).
Organize and layout the tips in tip-boxes for spotting (see Notes for suggestions).
Place the base plate on the pipette alignment tool. Ensure the screws that level the alignment tool fit into the three recessed holes in the base plate (Figure 3A).
Note: The following steps (E7-E10) should be performed in a Biological Safety Cabinet over an absorbent pad soaked in a tuberculocidal agent.
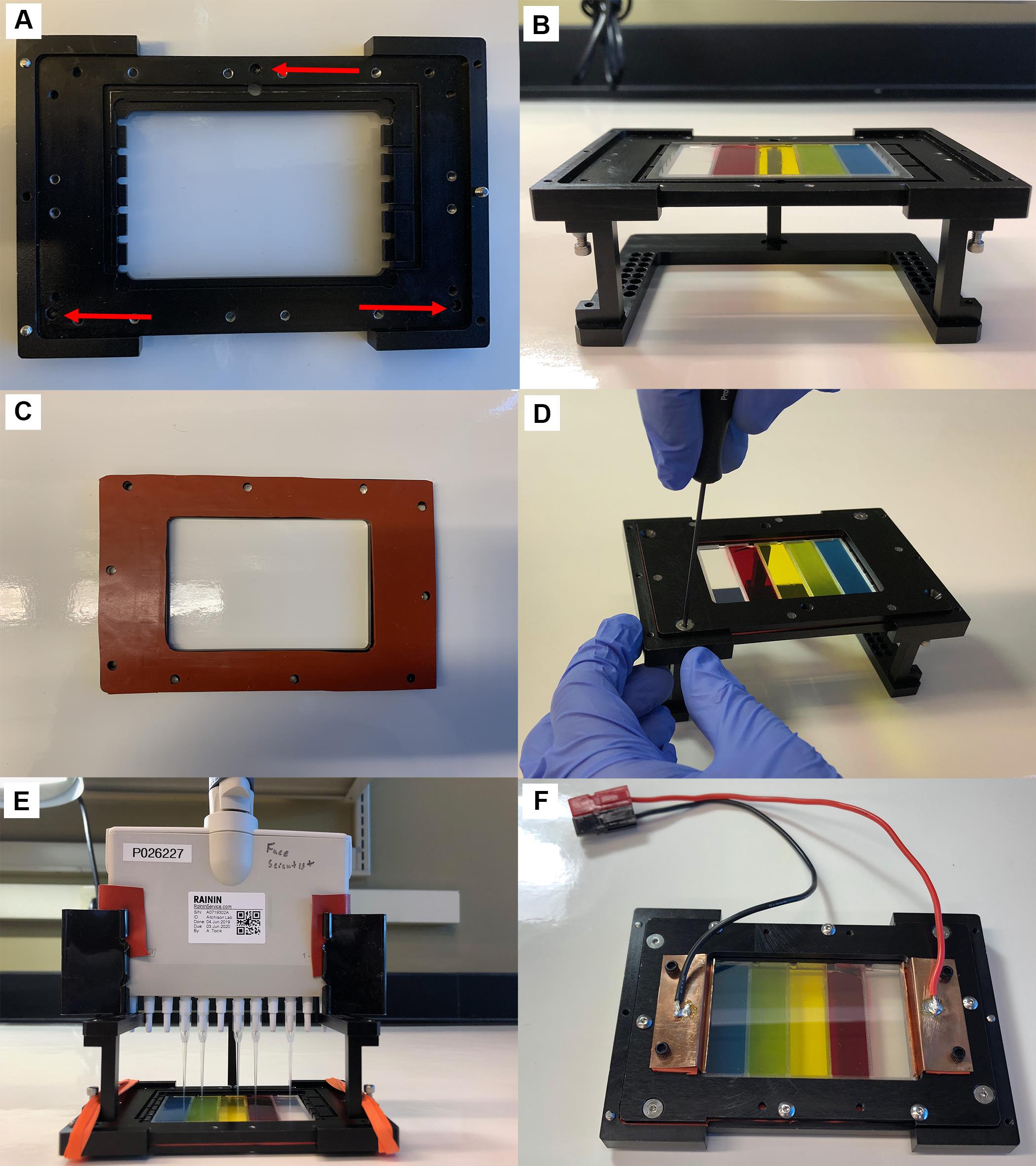
Figure 3. Assembly of ODELAM chamber. A. The base plate has recessed holes for mounting the pipette alignment tool, as indicated by the red arrows. B. Place the agar slide in the recession. C-D. Place the clean silicon gasket on the bottom plate (C) and secure it with the appropriate button head and countersunk screws (D). E. Spot the cultures onto the agar pads using the correct tip sequence. F. Complete assembly of the chamber by adding the ITO slide, top plate, top gasket, and electrical contacts.Place the previously prepared agar slide in the recessed section of the ODELAM base plate (Figure 3B).
Place the bottom silicon gasket on the ODELAM bottom plate. Smooth out the gasket and ensure the screw holes are correctly aligned and the gasket has a minimal overhang around the edges of the bottom plate (Figure 3C).
Place the bottom plate onto the base plate and secure the two with the appropriate screws (Figure 3D).
Flip the assembly over and place the pipette alignment tool onto the base plate. Ensure the screws protruding from each leg are set into the three corresponding holes in the base plate.
Note: Be careful that the forward screw does not fall into the vent hole, as this sometimes happens and will cause the pipette tips to mark the agar.
Check the agar surface to see if the pipette tips mark the agar (Figure 3E). If they do, adjust the height of the alignment tool using the screws on the legs until the tips are within 1 mm of the surface. They should be close but not mark the media.
Use diagrams to guide spotting patterns for transferring from 96-well plate to ODELAM (see Notes)
Spot 0.8-1.1 μl of culture. If needed, increase or decrease the volume of culture spotted to adjust its diameter slightly.
Place the ITO glass slide into the recession in the base plate. Make sure the ITO conductive side of the slide is facing up.
Note: Mark the conductive side of the ITO slide with an alcohol-resistant marker to identify the conductive side.
Place the upper silicon gasket onto the upper plate (similar to Step E6, Figure 3C). Ensure the gasket does not overhang the center hole in the plate as this could interfere with the electrical contacts to the ITO slide.
Secure the top plate to the base plate with screws.
Screw in the vent plugs on the bottom plate.
Disinfect the ODELAM chamber by wiping with a disinfectant-soaked towel but avoid touching or getting disinfectant on the ITO slide as this will cause condensation that will interfere with imaging.
Attach the electrodes and silicon gasket electrode spacers with the plastic screws to the top plate and flip the chamber over (Figure 3F).
Keeping track of plate orientation, place the assembled chamber onto the microscope stage.
Connect electrodes to the power supply, turn on the heater power supply, and ensure that the current flows through the ITO slide. If the electrodes are in contact with aluminum, the power supply may indicate a short or have a high current. Look for a current from 0.15 A to 0.2 A at 10 V.
Proceed to the next section on ODELAM microscope control.
ODELAM microscope control
Open the Visual Studio Code.
Create a python terminal and activate the odelay virtual environment.
Run the python file ODELAY microscopecontrol.py (e.g., python ODELAYmicroscopecontrol.py).
Select the appropriate directories, files, and ODELAY experiment type for the experiment.
Once the new graphical user interface has loaded, check to ensure the ROI layout on the microscope control panel matches that of the desired experiment (Figure 4).
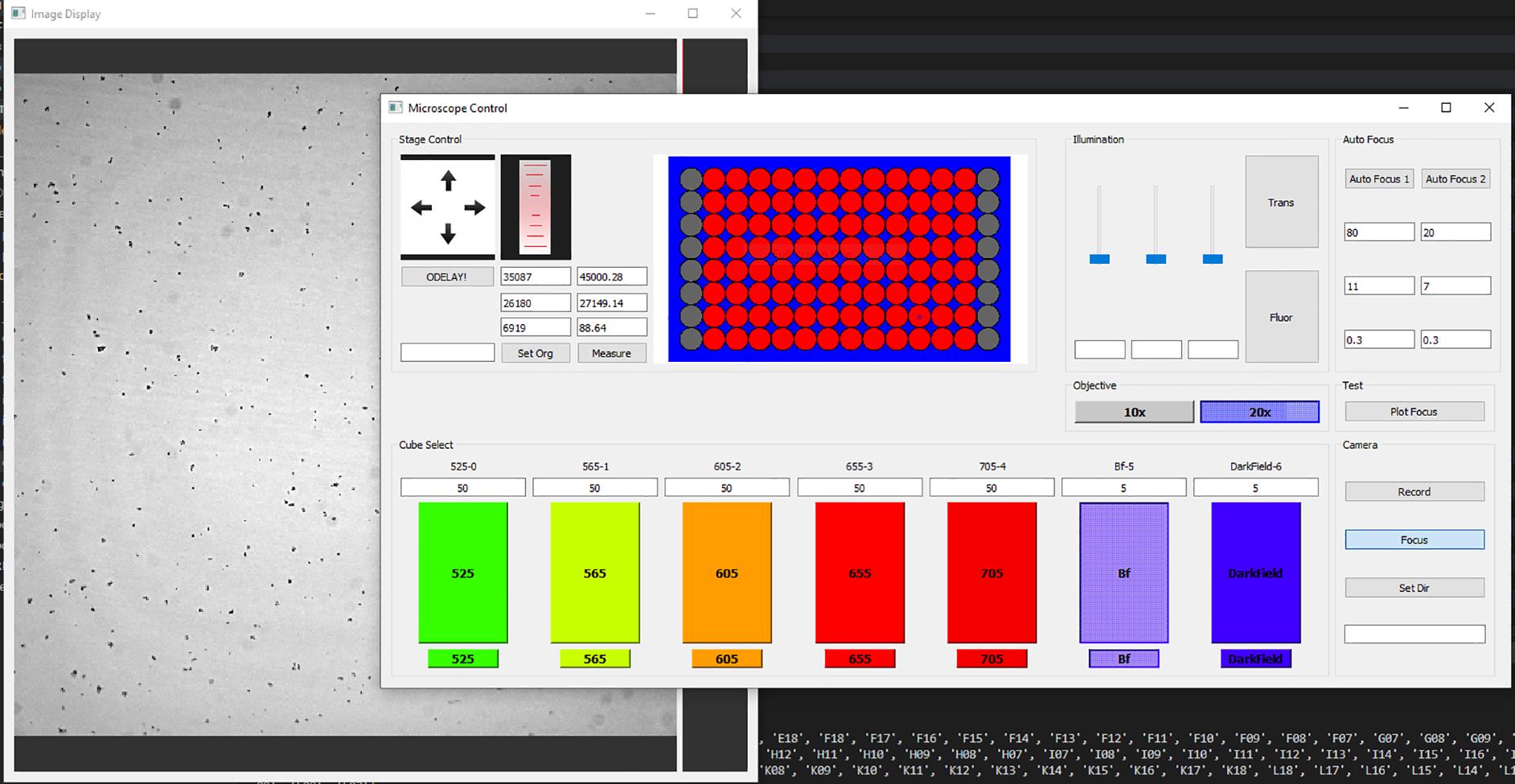
Figure 4. ODELAM microscope control interface and image display. The ODELAM microscope interface consists of a microscope control panel and an image display panel. An image of mycobacterium is displayed. The red circles in the control panel center indicate the ROIs that will be recorded, and the grey circles indicate the ROIs that will be ignored. Autofocus parameters for phase 1 and phase 2 are indicated and editable on the right. Camera exposure times are listed above the fluorescent cube selected.Start the camera by pressing the focus button.
Move the stage to a spot in the upper left corner of the array, where cells are present.
Focus the image and then move the upper left most ROI on the array (Spot E06 is the origin).
Press the set origin button.
Move to a ROI close to a leveling screw that has cells to focus on and use the leveling screw to focus the image.
Repeat this step for all three leveling screws until the image is in focus at all three ROIs next to the leveling screws.
Evaluate other ROIs and use the Autofocus 1 button to evaluate if those can be brought into focus by the default Autofocus parameters. Increase or decrease the Autofocus as needed to ensure all ROI can be found by the Autofocus settings (see Notes for additional Instructions).
Press the ODELAY button to begin recording data.
ODELAM Chamber Sterilization
Fill two small plastic containers (e.g., tip box lids) and one the size of the ODELAM chamber with LoPhene. Prepare the biological safety cabinet with LoPhene-soaked absorbent pads as a working surface.
After the experiment, remove the chamber from the microscope. Check for leaks. If necessary, apply disinfectant.
Remove the copper contacts using the 3/32 hex driver and place the contact and screws in an appropriate storage container.
Move the chamber to the biological safety cabinet.
Use the 1/16-inch driver to remove the 10 flat head and button head screws from the bottom plate.
After removing each screw, drop them into one of the two tip box lids with LoPhene.
Gently but firmly, pry the bottom plate and gasket from the base plate. Do not use a tool for this as it could damage the gasket or either plate. Take your time as the silicon will stick to two plates together but will slowly release with firm but constant pressure.
Gently pry the agarose slide from the gasket and place the slide in the second tip box filled with LoPhene. Make sure LoPhene completely covers the agar. Usually, some condensation forms on the edges of the slide at this point. Avoid touching it and clean the gloves thoroughly as they can become contaminated with Mtb at this point.
Completely separate the bottom gasket from the bottom plate and place both in the plastic container filled with LoPhene. Make sure each part is covered.
Repeat Steps G5-G7 for the top plate. Gently but with constant and firm pressure, pry the top plate and top gasket from the base plate.
Remove the ITO coated slide and place it in the tip box lid with the screws. Ensure the ITO slide is immersed in LoPhene.
Separate the top gasket from the top plate and the top plate, top gasket, and base plate into the plastic container with LoPhene. Add extra LoPhene if needed to cover all parts completely.
After approximately 15-20 min, remove the agarose slide from the LoPhene. Place the agarose into a solid waste disposal within the hood and glass slide in the sharp container.
Dump the LoPhene that the agarose slide was in into a liquid waste container.
Spray LoPhene on the other containers and remove them from the biological safety cabinet.
Wash all parts and screws with generous amounts of deionized water to remove the LoPhene. Finally, spray all parts with 70% ethanol and dry with a paper towel.
Store the disassembled chamber for future use.
Data analysis
Note: This section assumes that all data analysis is performed with the ODELAYTools python package executed in the command line and utilizing High Performance Compute cluster as described in the ODELAY ReadMe.md file. Please follow the instructions there to install the software and package commands. Additional features to perform analysis on standalone desktops are in development.
Activate the ODELAY python environment (e.g., >conda activate “name of odelay environment”).
Create a data directory where the processed data of the experiment will be written.
Set the image directory where the microscope images were written using the command “odelay set-image-dir”, and enter the path to the image directory at the command prompt.
Set the data directory using the command “odelay set-data-dir”.
Initialize the data processing with “odelay initialize”. Wait for the response that the experiment is initialized.
Finally, enter the command prompt >odelay process all.
Make sure that a *Spot-Layout.xlsx file is correctly filled out and in the data directory.
After the data has finished processing, enter the command >odelay summarize-experiment. This command will reduce the dataset to a single hdf5 file.
Plot summary histograms of all regions of interest using the command >odelay plot-summary Mtb (Figure 5A).
Plot growth curves for histograms with the command >odelay plot-gc all Mtb. This will plot all regions of interest successfully processed in the experiment (Figure 5B).
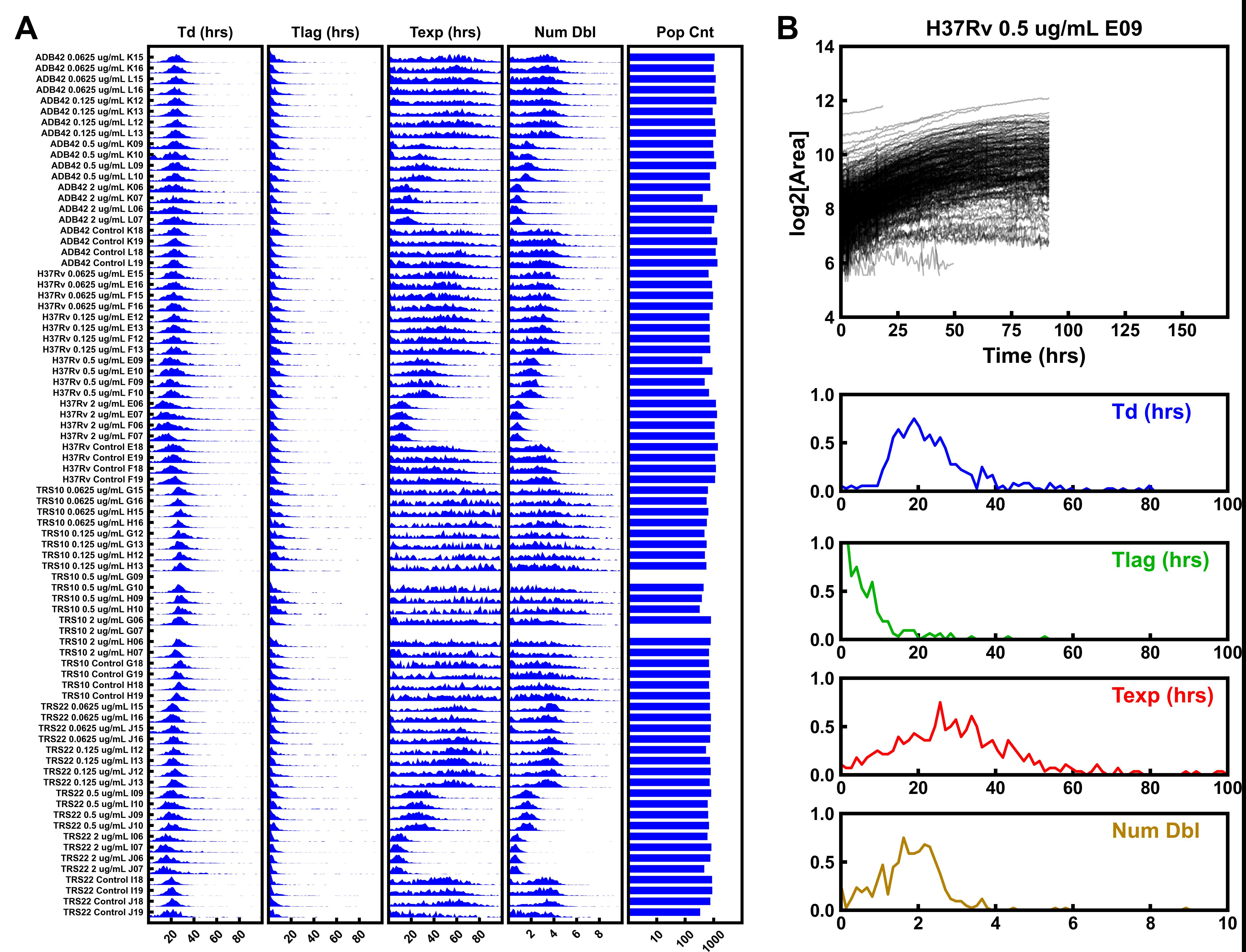
Figure 5. Example data from ODELAM experiments. Summary histograms for each region of interest in an ODELAM experiment. A. From left to right are histograms for the kinetic parameters of doubling time (Td), lag time (Tlag), time in exponential phase (Texp), and number of doublings (Num Dbl). The total population given on a Log10 scale is in the rightmost column. B. Plot of CFU growth curves observed in ROI E09. Histograms for doubling time, lag time, exponential time, and number of doublings are shown and can be inspected.
Notes
Layout Patterns for 96-Well plates
ODELAM spotting requires practice and organization to transfer samples from a 96-well plate to the 384-well pitch of the ODELAM plate. The following examples describe two strategies for arranging samples in a 96-well source plate. The first example shows how to spot a 96-well plate.
Arrange the 96-well plate according to how many samples are required. Usually, multiples of four are best.
Arrange the tips in the tip boxes according to the number of conditions required for the configuration of the agar pads (Figure 6A).
Alternate from left to right every row when spotting to generate a checkerboard pattern on the agarose slide (Figure 6B).
Repeat step 3 for the rows that were skipped in the previous step (Figure 6C).
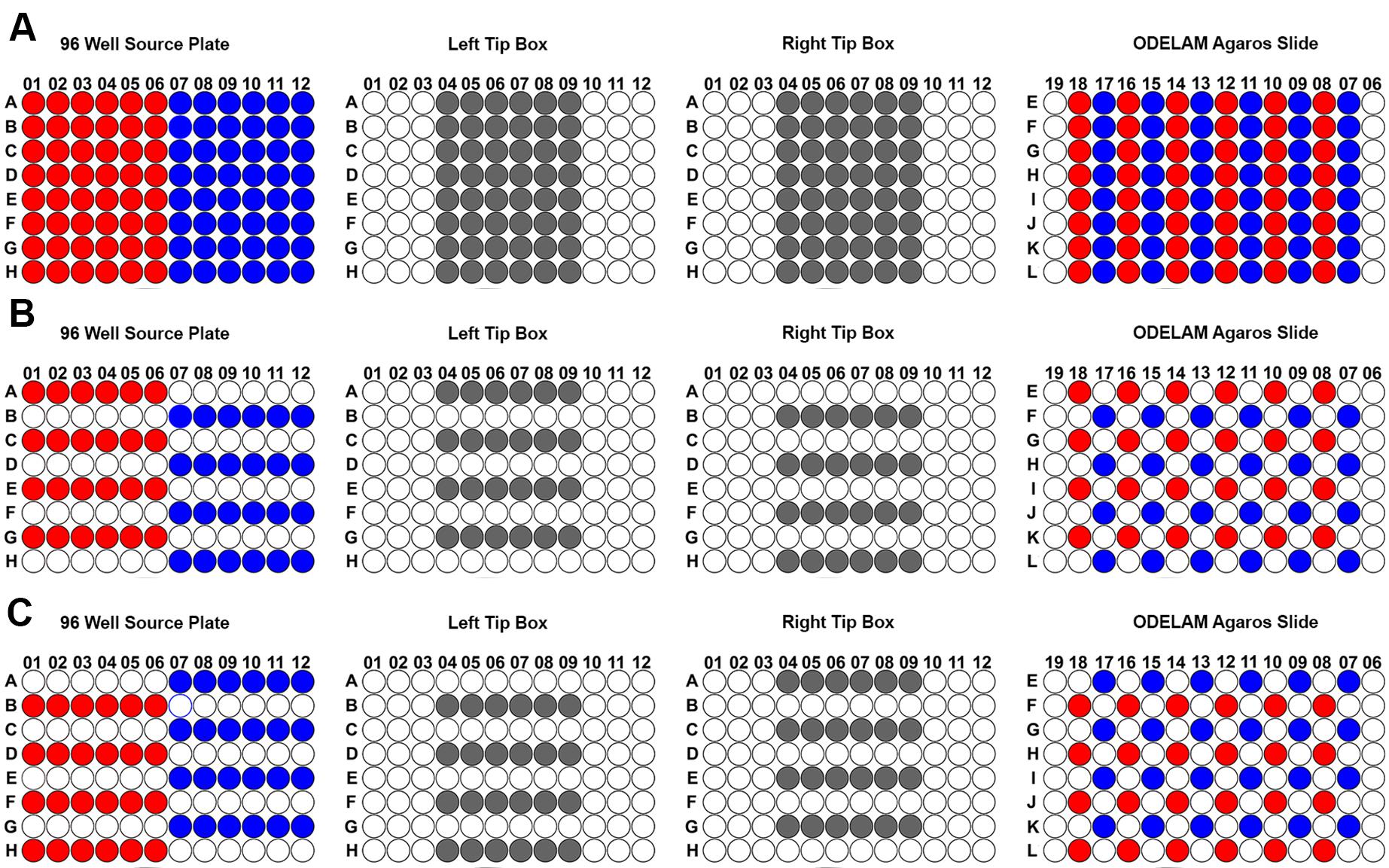
Figure 6. 96-well layout patterns for ODELAM experiments. A. Samples from a 96-well source plates (left) are transferred to the ODELAM agarose slide using the patterns shown in panels B and C to arrive at the final patterning on the top right. B. First step in patterning, selecting the samples shown on the left using the tip box patterns shown in the middle (grey) to produce the pattern on the agarose slide shown on the right. It is important to alternate every other row to prevent cultures from mixing while spotting. C. After the initial round of spots adsorb,spot the remaining wells as shown. Following these steps produces the interleaved pattern (top right) from the 96-well source plate (top left).Alternate patterns can be generated for spotting fewer colonies. A 12 or 24 sample pattern can be generated (Figure 7A) or for spotting on multiple conditions (Figure 7B).
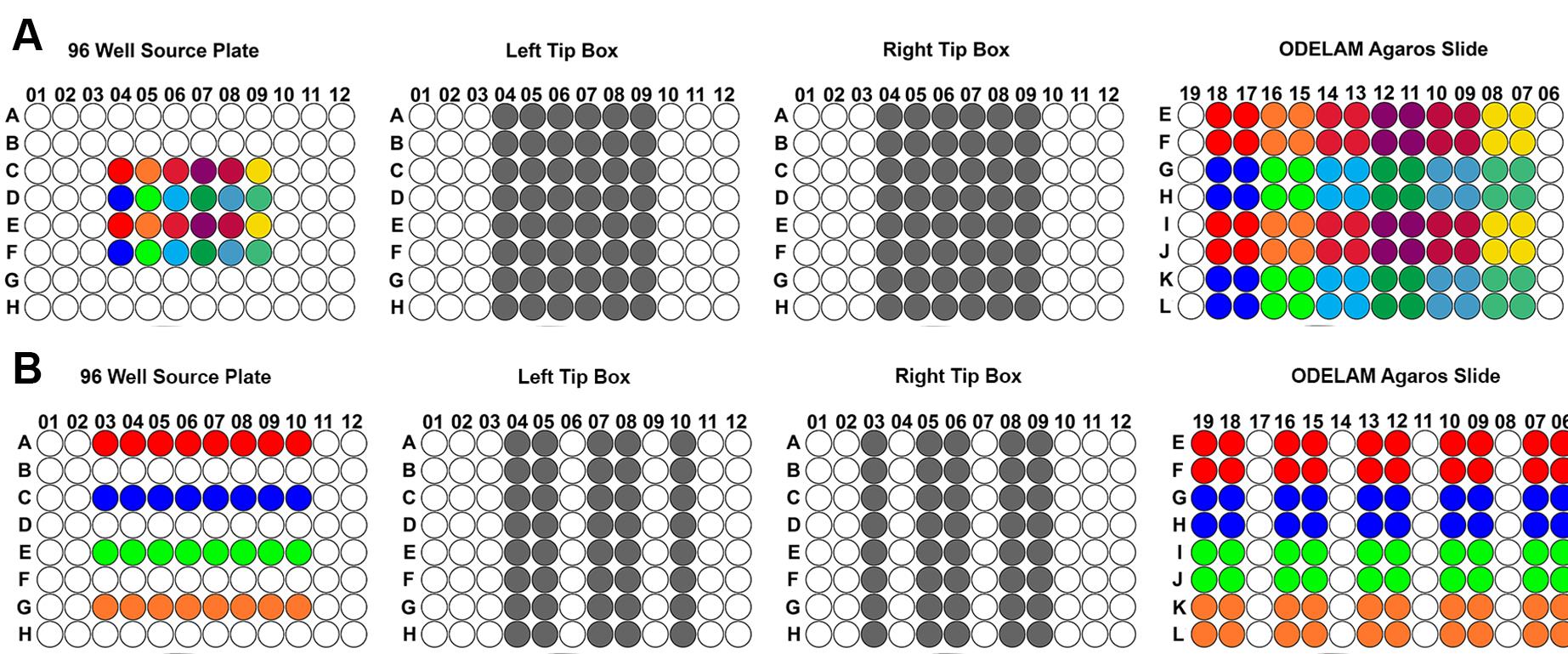
Figure 7. An example of 12 samples replicated eight times on a single condition. Each color represents a sample type. A. As described above, each row is sampled four times. B. Example with a five-condition four-sample layout. Four samples are arrayed across rows A, C, E, and G. Each is replicated four times per condition in the final layout. Pipette tips are marked using gray circles. Empty wells or tip positions are shown in white.Note: Columns 8,11,14, and 17 on the agarose slide are unused due to the presence of the gasket dividing the agar pads (refer to Figures 2 and 3 for gasket geometry).
Autofocus Settings
The autofocus settings are flexible to ensure that all spots will stay in focus throughout the experiment. There are two autofocus settings, Autofocus 1 and Autofocus 2.
Autofocus 1 is utilized to find focus for the first timepoint of an ROI.
Thereafter, Autofocus 2 uses settings that increase the speed of image acquisition.
When setting Autofocus 1, ensure all ROIs are within ± range setting.
With larger ranges, it is important to increase the number of images collected so that the focus algorithm will converge on a good focus. Ideally, a good print of the gasket should enable ROI focus to stay within 60 μm of each other across the spotted array.
3D Printing Gaskets
Successfully printing 3D gaskets onto a microscope slide requires editing the G-code file that defines the printer movements. Most 3D printing software does not allow printing an object above the build plate that, in this case, is required to print on a 1 mm thick glass slide. TPU gasket 3D printing can be tricky and inconsistent. The printing speed, feed rates, extrusion temperature, and print cooling parameters that work will vary depending on the filament diameter and printer utilized. In general, a print head speed of 20 mm/s, removing filament retraction, an extruding temperature of 230°C, and a build plate temperature of 65°C are good starting points. We have found that drying the filament overnight at 70°C in an oven can dramatically increase print quality. This is due to water being absorbed into the polymer filament and then boiling out when the filament melts. The voids created from the water vaporizing make the extruded filament uneven, leading to flaws in the print. The following instructions give a general description of how to generate G-code files and edit the G-code so that the printer will print a gasket directly onto a glass slide. G-code files are text files with commands that tell the printer where and how to move the printing head. Correctly following these steps helps generate a gasket that allows molding of the agarose media for reliable time-lapse imaging.
Printing Slide Build Plate mount:
Generate a *stl file of a rectangular frame with an inner opening of approximately 50.75 mm × 75.75 mm and an outer measurement of 70 mm × 95 mm and thickness of 0.5 mm to 0.75 mm.
Center the frame on the build plate and generate the G-code file using standard PLA filament parameters. Do not use a raft or any support material.
Transfer the G-code file to the printer.
Print the frame on a glass plate and use Kapton tape around the outer edges of the frame to hold it down afterward. This frame will center the glass slide for printing the gasket and can be used several times. It is recommended to have a dedicated glass plate for printing slide gaskets that can be reliably removed and relocated on the build plate.
Import the gasket design (usually a *.stl file) into the 3D printer software (e.g., Cura3D).
Place the gasket on the center of the build plate.
Enter the appropriate printing parameters for TPU and generate the G-code file.
Open the ZchangerGcode.py file and change fileName variable to the G-code file generated in the previous step.
Note: Line 19 of the ZchangerGcode.py';TYPE:WALL-OUTER' may need to be changed to a different value depending on the printer software generating the G-code. Generally, there are notes added to the G-code. It may take some inspection of the file and trial and error to get this section correct.
Additionally, change the writeFileName to a name that is easy to recognize.
Run the file using >python ZchangerGcode.py. This will create a file where the z-height of the gasket is offset by 1.1 mm from the print bed, allowing the gasket to be printed on a 1 mm thick slide.
Transfer the file (named from writeFileName) the printer.
Place a 50 × 75 × 1 mm glass slide into the rectangular frame previously printed.
Tape the corners of the slide to the rectangular frame using Kapton tape.
Print the slide gasket.
Repeat steps 8-10 as necessary.
Recipes
7H9-GOT media (1,000 ml)
Weigh 4.7 g of 7H9 powder into a 1,000 ml glass media bottle
Add 900 ml of 18 MΩ H2O and dissolve the 7H9 powder
Add 4 ml of sterile 50% glycerol
Autoclave at 120°C for 30 min
Allow the liquid to cool to 55°C.
Add the following ingredients aseptically:
2.5 ml of sterile 20% Tween 80
100 ml of sterile OADC enrichment
10× 7H9-G media (41.6 ml)
Weigh 1.88 g of 7H9 powder into 50 ml Falcon tube
Add 40 ml of 18 MΩ H2O and dissolve the 7H9 powder by vortexing and slight heating
Add 1.6 ml of 50% glycerol
Filter sterilize using a 50 ml steriflip filter and conical tube
Acknowledgments
This project was funded by the National Institutes of Health (grant number, U19 AI106761 and NIH P41 GM109824).
Competing interests
The authors declare no competing interests.
References
- Aldridge, B. B., Fernandez-Suarez, M., Heller, D., Ambravaneswaran, V., Irimia, D., Toner, M. and Fortune, S. M. (2012). Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility.Science 335(6064): 100-104.
- Barr, D. A., Kamdolozi, M., Nishihara, Y., Ndhlovu, V., Khonga, M., Davies, G. R. and Sloan, D. J. (2016). Serial image analysis of Mycobacterium tuberculosis colony growth reveals a persistent subpopulation in sputum during treatment of pulmonary TB. Tuberculosis (Edinb) 98: 110-115.
- Choi, J., Yoo, J., Lee, M., Kim, E. G., Lee, J. S., Lee, S., Joo, S., Song, S. H., Kim, E. C., Lee, J. C., Kim, H. C., Jung, Y. G. and Kwon, S. (2014). A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci Transl Med 6(267): 267ra174.
- Dhar, N., McKinney, J. and Manina, G. (2016). Phenotypic Heterogeneity in Mycobacterium tuberculosis. Microbiol Spectr 4(6).
- Golchin, S. A., Stratford, J., Curry, R. J. and McFadden, J. (2012). A microfluidic system for long-term time-lapse microscopy studies of mycobacteria. Tuberculosis (Edinb) 92(6): 489-496.
- Herricks, T., Donczew, M., Mast, F. D., Rustad, T., Morrison, R., Sterling, T. R., Sherman, D. R. and Aitchison, J. D. (2020). ODELAM, rapid sequence-independent detection of drug resistance in isolates of Mycobacterium tuberculosis. Elife 9: e56613.
- Herricks, T., Dilworth, D. J., Mast, F. D., Li, S., Smith, J. J., Ratushny, A. V. and Aitchison, J. D. (2017a). One-Cell Doubling Evaluation by Living Arrays of Yeast, ODELAY! G3 (Bethesda) 7(1): 279-288.
- Herricks, T., Mast, F. D., Li, S. and Aitchison, J. D. (2017b). ODELAY: A Large-scale Method for Multi-parameter Quantification of Yeast Growth. J Vis Exp(125): 55879.
- Kim, S. J., Fernandez-Martinez, J., Nudelman, I., Shi, Y., Zhang, W., Raveh, B., Herricks, T., Slaughter, B. D., Hogan, J. A., Upla, P., Chemmama, I. E., Pellarin, R., Echeverria, I., Shivaraju, M., Chaudhury, A. S., Wang, J., Williams, R., Unruh, J. R., Greenberg, C. H., Jacobs, E. Y., Yu, Z., de la Cruz, M. J., Mironska, R., Stokes, D. L., Aitchison, J. D., Jarrold, M. F., Gerton, J. L., Ludtke, S. J., Akey, C. W., Chait, B. T., Sali, A. and Rout, M. P. (2018). Integrative structure and functional anatomy of a nuclear pore complex. Nature 555(7697): 475-482.
- Logsdon, M. M. and Aldridge, B. B. (2018). Stable Regulation of Cell Cycle Events in Mycobacteria: Insights from Inherently Heterogeneous Bacterial Populations. Front Microbiol 9: 514.
- Manina, G., Dhar, N. and McKinney, J. D. (2015). Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17(1): 32-46.
- Mast, F. D., Herricks, T., Strehler, K. M., Miller, L. R., Saleem, R. A., Rachubinski, R. A. and Aitchison, J. D. (2018). ESCRT-III is required for scissioning new peroxisomes from the endoplasmic reticulum.The J Cell Biol 217(6): 2087-2102.
- Pai, M., Behr, M. A., Dowdy, D., Dheda, K., Divangahi, M., Boehme, C. C., Ginsberg, A., Swaminathan, S., Spigelman, M., Getahun, H., Menzies, D. and Raviglione, M. (2016). Tuberculosis. Nature Rev Dis Primers 2: 16076.
- Rego, E. H., Audette, R. E. and Rubin, E. J. (2017). Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546(7656): 153-157.
- Santi, I., Dhar, N., Bousbaine, D., Wakamoto, Y. and McKinney, J. D. (2013). Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria. Nat Commun 4: 2470.
- Wakamoto, Y., Dhar, N., Chait, R., Schneider, K., Signorino-Gelo, F., Leibler, S. and McKinney, J. D. (2013). Dynamic persistence of antibiotic-stressed mycobacteria. Science 339(6115): 91-95.
- World Health Organization. (2020). Global Tuberculosis Report (2020).
Article Information
Copyright
Herricks et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Herricks, T., Donczew, M., Sherman, D. R. and Aitchison, J. D. (2021). ODELAM: Rapid Sequence-independent Detection of Drug Resistance in Mycobacterium tuberculosis Isolates. Bio-protocol 11(10): e4027. DOI: 10.21769/BioProtoc.4027.
- Herricks, T., Donczew, M., Mast, F. D., Rustad, T., Morrison, R., Sterling, T. R., Sherman, D. R. and Aitchison, J. D. (2020). ODELAM, rapid sequence-independent detection of drug resistance in isolates of Mycobacterium tuberculosis. Elife 9: e56613.
Category
Microbiology > Antimicrobial assay > Antibacterial assay
Microbiology > Microbial physiology > Adaptation
Biological Sciences > Microbiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









