- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Intracellular IRF5 Dimerization Assay
Published: Vol 11, Iss 10, May 20, 2021 DOI: 10.21769/BioProtoc.4021 Views: 6874
Reviewed by: Martin V KolevAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Computational Workflow for Membrane Protein–Ligand Interaction Studies: Focus on α5-Containing GABA (A) Receptors
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
Nov 20, 2025 2110 Views

SiMPull-POP: Quantification of Membrane Protein Assembly via Single Molecule Photobleaching
Ryan J. Schuck [...] Rajan Lamichhane
Jan 5, 2026 287 Views

Isolation of Antigen-Specific Nanobodies From Synthetic Libraries Using a Protein Selection Strategy That Combines MACS-Based Screening of YSD and FLI-TRAP
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
Jan 20, 2026 442 Views
Abstract
The intracellular interferon regulatory factor 5 (IRF5) dimerization assay is a technique designed to measure molecular interaction(s) with endogenous IRF5. Here, we present two methods that detect endogenous IRF5 homodimerization and interaction of endogenous IR5 with cell penetrating peptide (CPP) inhibitors. Briefly, to detect endogenous IRF5 dimers, THP-1 cells are incubated in the presence or absence of the IRF5-targeted CPP (IRF5-CPP) inhibitor for 30 min then the cells are stimulated with R848 for 1 h. Cell lysates are separated by native-polyacrylamide gel electrophoresis (PAGE) and IRF5 dimers are detected by immunoblotting with IRF5 antibodies. To detect endogenous interactions between IRF5 and FITC-labeled IRF5-CPP, an in-cell fluorescence resonance energy transfer (FRET) assay is used. In this assay, THP-1 cells are left untreated or treated with FITC-IRF5-CPP conjugated inhibitors for 1 h. Next, cells are fixed, permeabilized, and stained with anti-IRF5 and TRITC-conjugated secondary antibodies. Transfer of fluorescence can be measured and calculated as FRET units. These methods provide rapid and accurate assays to detect IRF5 molecular interactions.
Keywords: IRF5Background
Interferon regulatory factor 5 (IRF5) is a transcription factor that regulates pathogen-induced innate and acquired immune responses downstream of Toll-like receptor (TLR), retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and B cell receptor (BCR) (De et al., 2017; Thompson et al., 2018; Banga et al., 2020). IRF5 has been implicated in the pathogenesis of systemic lupus erythematosus due to its role in regulating the expression of proinflammatory cytokines such as IFN-α, IL-6, TNF-α, and IL-12 and pathogenic autoantibody production (Song et al., 2020). In an unstimulated condition, IRF5 is generally localized in the cytoplasm as a monomer (Thompson et al., 2018). Activation of the above receptors triggers cellular signaling cascades. IRF5 undergoes post-translational modification, which eventually leads to homodimerization, a critical event prior to nuclear translocation (Thompson et al., 2018). Here, we describe two methods designed for detecting IRF5 molecular interactions.
A native-PAGE method is used to detect endogenous IRF5 homodimers. THP-1 cells are incubated with or without (1 and 10 μM) IRF5-CPP inhibitors for 30 min, and subsequently stimulated with 1 μM of R848 (a TLR7 ligand) or left unstimulated for 1 h. Next, cell lysates are run on native polyacrylamide gel electrophoresis (PAGE) and IRF5 dimerization is detected by immunoblotting with IRF5 and HRP-conjugated secondary antibodies. The intracellular fluorescence resonance energy transfer (FRET) assay is used to detect binding of endogenous IRF5 to FITC-IRF5-CPP conjugated inhibitors through a FRET signal (Banga et al., 2020; Song et al., 2020). FRET is a technique designed for detecting molecular interaction in which the excited molecule (the donor) transfers non-radiative energy to another molecule (the acceptor) within a distance of ~1-10 nm (Doucey et al., 2003; Ujlaky-Nagy et al., 2018; Banga et al., 2020). THP-1 cells are incubated with or without (1 and 10 μM) IRF5-CPP inhibitors for 1 h. The untreated and FITC-IRF5-CPP- treated THP-1 cells are then fixed, permeabilized, and stained with anti-IRF5, anti-IRF3, or anti-IRF7 (other IRF family members) antibodies and TRITC secondary antibodies. Cell-associated fluorescence is measured on a BioTek Synergy Neo2 at 525 nm upon excitation at 488 nm (E1), at 600 nm upon excitation at 540 nm (E2), and at 600 nm upon excitation at 488 nm (E3). The transfer of fluorescence is calculated as FRET units as follows: FRET unit = (E3both − E3none) − ([E3TRITC − E3none) × (E2both/E2TRITC]) − ([E3FITC − E3none] × [E1both/E1FITC]) (Doucey et al., 2003; Banga et al., 2020; Song et al., 2020). The different fluorescence values (E) were measured on unlabeled cells (Enone) or cells labeled with FITC (EFITC) and TRITC (ETRITC) (Banga et al., 2020; Song et al., 2020).
These techniques can be broadly applied to evaluate intracellular molecular interactions in living cells through pharmacological and molecular studies. They provide a rapid and reliable method of screening molecular interactions, which require standard equipment that are readily available in almost every laboratory.
Part I. Native-PAGE (Polyacrylamide Gel Electrophoresis)
Materials and Reagents
THP-1 cells (ATCC, catalog number: TIB-202)
RPMI-1640 medium (Gibco, catalog number: 11875093)
Fetal bovine serum (Gibco, catalog number: 16000044)
Penicillin-streptomycin (Gibco by Life Technologies, catalog number: 15140122)
IRF5 cell penetrating peptides (IRF5-CPP) and FITC-IRF5-CPP inhibitors (Hoffman-LaRoche, Patent number WO2014001229A2)
Resiquimod (R848) (Millipore Sigma, catalog number: SML0196)
Sodium deoxycholate (DOC) (Thermo Fisher, catalog number: 89904)
DC protein assay (Bio-Rad, catalog number: 5000111)
BCA protein assay (Thermo Fisher, catalog number: 23227)
Anti-IRF3, rabbit monoclonal antibody (Abcam, catalog number: ab76409)
Anti-IRF5 (Cell Signaling Tech, catalog number: 3257, rabbit polyclonal antibody or catalog number: 13496, rabbit monoclonal antibody)
Anti-IRF7, rabbit polyclonal antibody (Cell Signaling Tech, catalog number: 4920)
Horseradish peroxidase (HRP)–conjugated anti-β-actin, rabbit monoclonal antibody (Cell Signaling Tech, catalog number: 5125)
Anti-rabbit IgG HRP-conjugated secondary antibody (Cell Signaling Tech, catalog number: 7074)
NP40 cell lysis buffer (Invitrogen, catalog number: FNN0021)
Phenylmethylsulfonyl fluoride (PMSF) (Millipore Sigma, catalog number: 10837091001)
Protease inhibitor (Millipore Sigma, catalog number: P-2714)
Native gel running buffer, Tris-glycine buffer 10× concentrate, pH 8.3 (Millipore Sigma, catalog number: T4904-1L)
Glycerol (Millipore Sigma, catalog number: G9012)
Immobilon-P 0.45 μm PVDF membrane (Millipore, catalog number: IPVH00010)
Bovine serum albumin (BSA) (Millipore Sigma catalog number: 05470-5G)
Nonfat-dried milk bovine (Millipore Sigma, catalog number: M7409-1BTL)
Bromophenol blue (Millipore Sigma, catalog number: B5525)
30% acrylamide/Bis solution, 37.5:1, 500 ml (Bio-Rad, catalog number: 161-0158)
Resolving gel buffer, 1.5 M Tris-HCl, pH 8.8, 1 L (Bio-Rad, catalog number: 161-0798)
Stacking gel buffer, 0.5 M Tris-HCl, pH 6.8, 1 L (Bio-Rad, catalog number: 161-0799)
SDS solution, 10% (w/v), 250 ml (Bio-Rad, catalog number: 161-0416)
Pierce 20× TBS Tween 20 buffer (TBST) (Thermo Fisher, catalog number: 28360)
Western bright ECL HRP substrate (Advansta, catalog number: K-12045-C20)
Precision plus protein standard (Bio-Rad, catalog number: 161-0374) in which high molecular weight bands can be detected on native gel
Equipment
ChemiDoc MP Imaging System (Bio-Rad, catalog number: 17001402)
Mini-Transblot Cell and PowerPac Basic Power Supply (Bio-Rad, catalog number: 1703989)
Mini-Protean Tetra Vertical Electrophoresis Cell for Mini Precast Gels (Bio-Rad, catalog number: 1658005)
Procedure
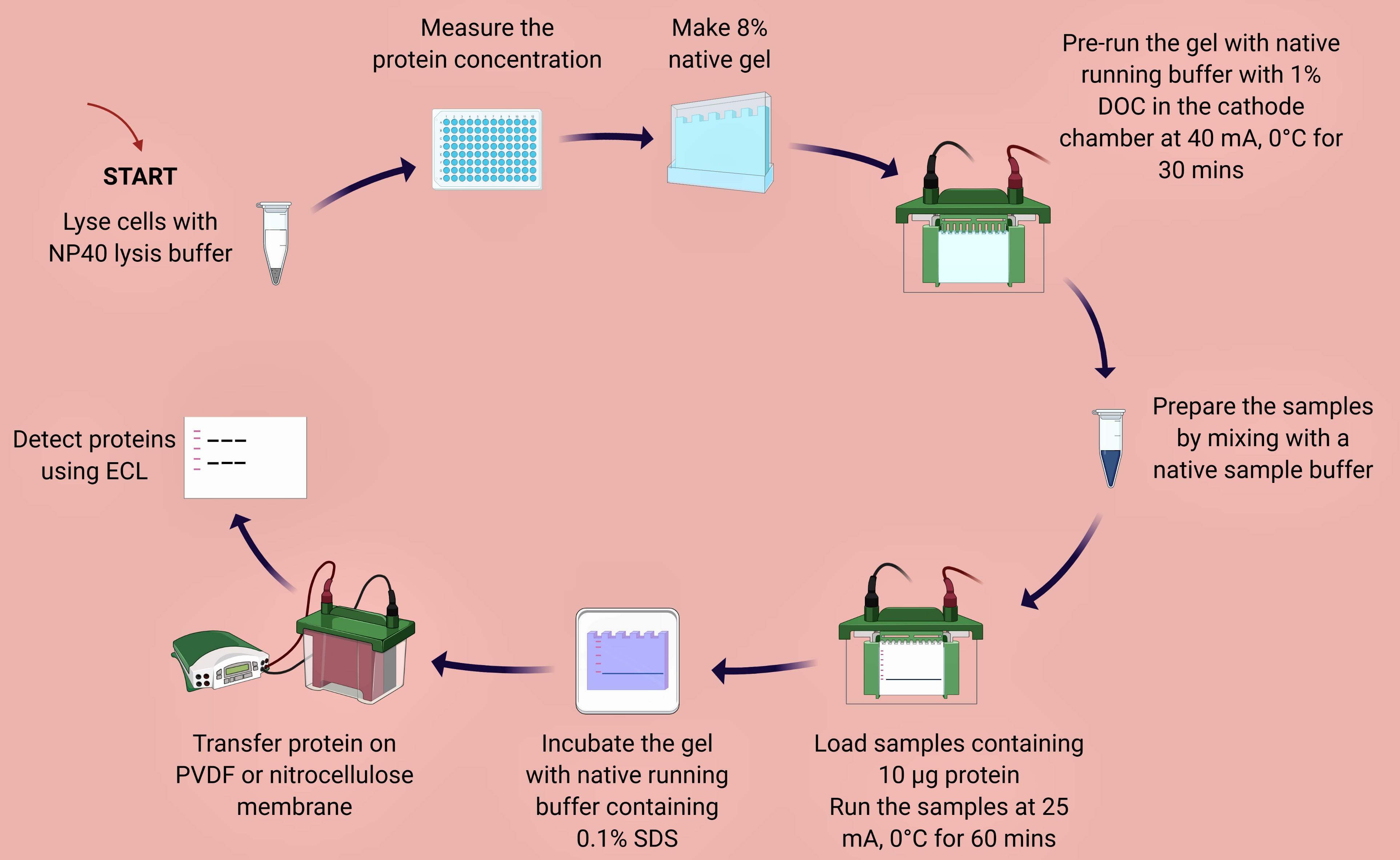
Figure 1. Overview of native-PAGE method designed for detecting endogenous IRF5 homodimers
R848-induced IRF5 homodimerization and inhibition of IRF5 homodimerization by IRF5-CPPs in THP-1
Plate THP-1 cells (6 × 106/ml) in complete RPMI-1640 medium in a 6-well plate and keep in the incubator (37°C, 5% CO2) for 24 h.
Prepare 1 and 10 µM of IRF5-CPP and 1 µM of R848 in complete RPMI-1640 medium. Incubate THP-1 cells with or without final concentration of IRF5-CPP for 30 min. Subsequently add 1 µM of R848 and incubate at 37°C, 5% CO2 for 1 h.
Transfer cells and media containing 6 × 106 THP-1 cells into a 5 ml or 15 ml tube.
Centrifuge samples at 400 × g, 4°C for 5 min. Aspirate supernatant without disturbing the cell pellet.
Wash cells with 5 ml PBS and centrifuge at 400 × g, 4°C for 5 min. Aspirate PBS without disturbing the cell pellet.
Cell Lysis and Protein Concentration Measurement
Prepare cell lysis buffer by adding 1 mM PMSF and 500 μl of 10× protease inhibitor cocktail to 5 ml NP40 lysis buffer immediately prior to use. (NP40 lysis buffer, instructions from the manufacturer: http://tools.thermofisher.com/content/sfs/manuals/FNN0021_Rev%200908.pdf)
Lyse the cell pellet in NP40 cell lysis buffer for 30 min, on ice, and vortex every 10 min. Add 500 μl of NP40 cell lysis buffer to the cell pellet. (The volume of cell lysis buffer depends on the cell number and expression of target protein.)
Transfer the extract to microcentrifuge tubes and centrifuge at 8,000 × g for 10 min at 4°C.
Aliquot the clear lysate to clean microfuge tubes. These samples are ready for BCA or DC protein assay. Lysates can be stored at -80°C. Avoid multiple freeze/thaws.
Measure the protein concentration using BCA or DC protein assay as per kit instruction. Keep samples on ice to prevent protein denaturation.
8% Native Polyacrylamide Gel Hand Casting
Make 8% Native Gel (as per Bio-Rad Casting Instruction, http://www.bio-rad.com/webroot/web/pdf/lsr/literature/4110106B.pdf).
Note: Substitute SDS with deionized distilled water. SDS is a known amphipathic surfactant that denatures proteins. Do NOT use SDS except for gel incubation post-native PAGE.
Native-PAGE and Immunoblotting
Put pre-running native running buffer (25 mM Tris and 192 mM glycine, pH 8.3) in the freezer until it reaches 0°C temperature. Make sure that there are no ice crystals prior to pouring into the electrophoresis chamber unit.
Pre-run native gel with native running buffer with 1% DOC in the cathode chamber, and native running buffer in the anode chamber at 40 mA, 0°C for 30 min. Keep electrophoresis unit on ice and maintain 0°C temperature (Figure 2).
Prepare samples for native gel electrophoresis by mixing the sample with native sample buffer. The final protein concentration per sample is 10 µg/well. Do NOT heat the samples.
NOTE: Heating will denature the proteins which will lead to dissociation of IRF5 dimers.
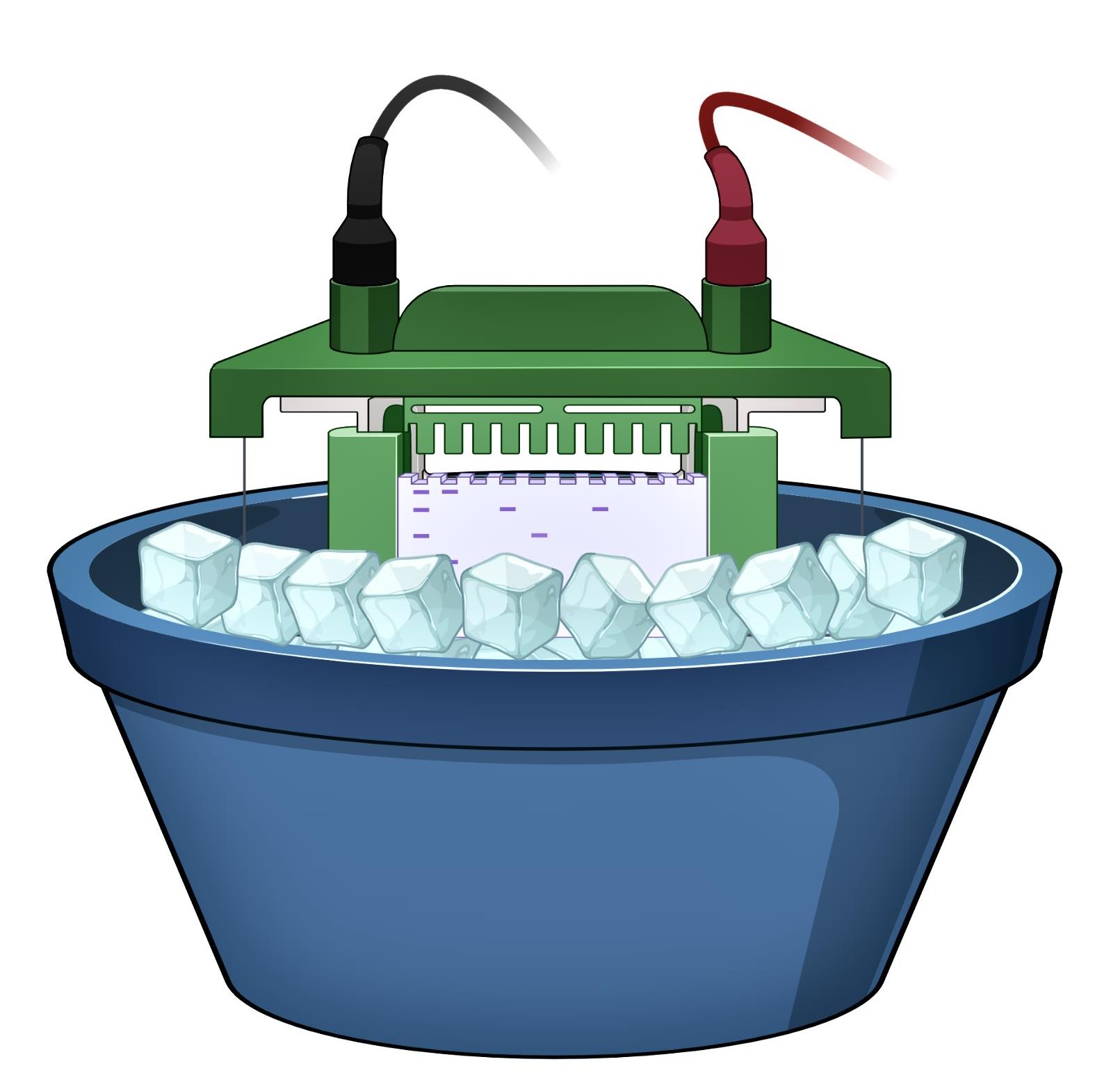
Figure 2. Schematic diagram of native-PAGE set upAdd fresh cold native running buffers in the anode and cathode chambers (with 1% DOC in the cathode). Keep electrophoresis unit on ice and maintain at a temperature of 0°C (Figure 2).
Load the ladder and samples (total protein concentration: 10 µg per sample per well).
Electrophorese samples at 25 mA, 0°C, for 60 min or until you see the dye close to the bottom.
Incubate gel in native running buffer containing 0.1% SDS for 30 min.
NOTE: Dilute 10% SDS in deionized distilled water. The addition of 0.1% SDS is to improve transfer efficiency.
Proceed to protein transfer on PVDF or nitrocellulose membrane.
Pre-wet the membrane and place the gel/membrane sandwich into the transfer apparatus.
Add fresh transfer buffer to the tank.
Run at 100 V for 60-70 min while keeping the tank on ice.
After transfer, air-dry the PVDF membrane.
Briefly soak in 100% methanol to wet the membrane.
Wash the membrane with distilled water 3× to remove the methanol.
Block the membrane with 5% non-fat milk-TBST or 5% BSA-TBST solution for 1 h.
NOTE: Dilute 20× TBST in distilled water to make 1× TBST.
Wash the membrane 3× with TBST.
Probe the membrane with primary antibody (IRF5, IRF3 or IRF7) on a rocker at 4°C overnight.
Wash the membrane 3× with TBST.
Probe the membrane with HRP-conjugated secondary antibody on a rocker at room temperature for 1 h.
Wash the membrane 3× with TBST.
Proceed with chemiluminescent detection of proteins using ChemiDoc Imaging System.
The resulting immunoblot (Figure 3) shows that R848 treatment increased endogenous IRF5 dimer (IRF5)2 formation and that IRF5-CPP5 and not IRF5-CPP2 was able to inhibit dimerization by R848. The IRF5 monomer (IRF5) and β-actin (loading control) are shown.
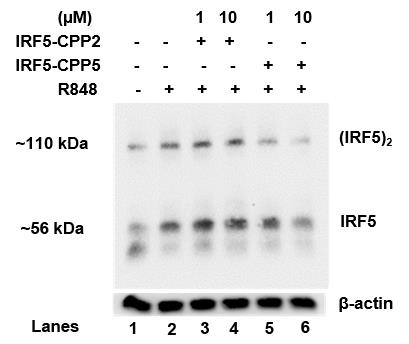
Figure 3. Native-PAGE. On lane 1 control – untreated THP-1, lane 2 treated with 1 μM R848, lane 3 treated with 1 μM IRF5-CPP2 and 1 μM R848, lane 4 treated with 10 μM IRF5-CPP2 and 1 μM R848, lane 5 treated with 1 μM IRF5-CPP5 and 1 μM R848, and lane 6 treated with 10 μM IRF5-CPP5 and 1 μM R848.
Recipes
Native Sample Buffer
To make 10 ml of Native Sample Buffer, mix 15% glycerol (v/v), 1% DOC (w/v), and 1.25 ml of 0.5 M Tris-Cl, pH 6.8 and 0.1% bromophenol blue (w/v) and deionized distilled water.
Native Running Buffer
To make 1 L of Native Running Buffer, mix 100 ml of 10× Tris-Glycine Buffer (pH 8.3) and 900 ml of deionized distilled water.
Transfer Buffer
To make 1 L of Transfer Buffer, add 100 ml of 10× transfer buffer, 100 ml of methanol and 800 ml of deionized distilled water.
Complete RPMI-1640 Medium
Mix the following: a bottle of 500 ml RPMI-1640, 10% FBS, 1% Penicillin-Streptomycin
Part II. In-cell Fluorescence Resonance Energy Transfer (FRET)
Materials and Reagents
Corning 96-well black plates (Fisher Scientific, catalog number: 07200627)
5 ml macrotubes (Fisher Scientific, catalog number: 501537789)
THP-1 cells (ATCC, catalog number: TIB-202)
RPMI-1640 Medium (Gibco, catalog number: 11875093)
Fetal Bovine Serum (Gibco, catalog number: 16000044)
Penicillin-Streptomycin (Gibco by Life Technologies, catalog number: 15140122)
FITC-IRF5-CPP inhibitors (Hoffman-LaRoche, Patent WO2014001229A2)
Resiquimod (R848) (Millipore Sigma, catalog number: SML0196)
Foxp3 Fixation/Permeabilization Buffer (Invitrogen, catalog number: 00-5523-00)
Bovine serum albumin (BSA) (Millipore Sigma catalog number: 05470-5G)
Permeabilization buffer (Invitrogen, catalog number: 00-5523-00)
Anti-IRF5, rabbit monoclonal antibody (Abcam, catalog number: ab124792)
Goat anti-rabbit tetramethyl rhodamine isothiocyanate (TRITC) antibody (Abcam, catalog number: ab6718)
Equipment
Biotek Synergy Neo2 (Biotek, model: BTNEO2) or any similar plate reader
Procedure
Buffer and Solution Preparation
Prepare fresh Foxp3 Fixation/Permeabilization working solution by mixing 1 part of Foxp3 Fixation/Permeabilization concentrate with 3 parts of PBS containing 2% BSA. For 3-5 million cells, 1 ml of the working solution is required for each sample.
Prepare 1× working solution of Permeabilization Buffer by mixing 1 part of 10× Permeabilization Buffer with 9 parts of deionized distilled water. 1 ml of the working solution is required for each sample.
Detection of endogenous IRF5 interaction with FITC-IRF5-CPP inhibitors in THP-1
Plate 2 × 105-4 × 105/ml THP-1 cells in complete RPMI-1640 media in a 96-well plate (in triplicate per condition/treatment) and keep in the incubator (37°C, 5% CO2) for 24 h.
Prepare 1 and 10 µM of FITC-IRF5-CPP conjugated inhibitors in complete RPMI-1640 medium. Aspirate medium and incubate THP-1 cells with or without FITC-IRF5-CPP in 37°C, 5% CO2 for 1 h.
NOTE: Keep the samples protected from light.
Transfer the FITC-IRF5-CPP treated cells in to a 5 ml tube.
Centrifuge at 400 × g, 4°C for 5 min. Aspirate supernatant without disturbing the cell pellet.
Wash cells with 3 ml PBS and centrifuge at 400 × g, 4°C for 5 min. Aspirate PBS without disturbing the cell pellet.
Add 1 ml of Foxp3 Fixation/Permeabilization working solution to each tube and pulse vortex.
Incubate for 30-60 min at room temperature or for up 18 h at 2-8°C.
Cells should have settled in the bottom after 18 h. Carefully aspirate supernatant. For short incubation (30-60 min at room temperature), spin at 400 × g, 4°C for 5 min.
Add 1 ml of 1× Permeabilization Buffer to each tube and incubate for 30 min at room temperature.
Spin at 400 × g for 5 min.
Resuspend pellet in 50 µl residual volume of 1× Permeabilization Buffer.
[Optional] Block with 10% BSA by adding 5 µl directly to each tube. Incubate for 15 min at room temperature.
Without washing, add the recommended amount of primary (anti-IRF5) antibody for detection of intracellular antigen and incubate for at least 30 min at room temperature. Protect from the light.
Wash with 3 ml of PBS.
Repeat Steps B9-B12 and add the recommended amount of secondary (TRITC) antibody (to bind to anti-IRF5 antibody).
Spin at 400 × g for 5 min. Aspirate supernatant leaving 30 µl residual volume.
Add 30 µl of 4% PFA and resuspend stained cells. Protect from the light.
Transfer samples to a 96-well plate. Prior to reading, add PBS to a final volume of 150-200 µl.
NOTE: Make sure you have the same number of cells per well. Counting the cells is highly recommended.
Measure intracellular fluorescence on Biotek Synergy Neo2 (Bioteck, VT, USA) or any similar plate reader at 525 nm upon excitation at 488 nm (E1), at 600 nm after excitation at 540 nm (E2), and 600 nm after excitation at 488 nm (E3).
Set up the machine with 3 spectra:
FITC 488 excitation 525 emission
TRITC 540 excitation 600 emission
FRET 488 excitation 600 emission
The transfer of fluorescence was calculated as FRET units as follows: FRET unit = (E3c − E3none) − ([E3TRITC − E3none) × (E2none/E2TRITC]) − ([E3 FITC −E3none] × [E1both/E1TRITC ]). The different fluorescence values (E) were measured on unlabeled cells (Enone) or cells labeled with FITC (EFITC) and TRITC (ETRITC).
The resulting bar graph (Figure 4) shows an increase in FRET signal (unit) between endogenous IRF5 and FITC-IRF5-CPP2 or FITC-IRF5-CPP5 but not FITC-IRF5-CPP8 or –CPP9.
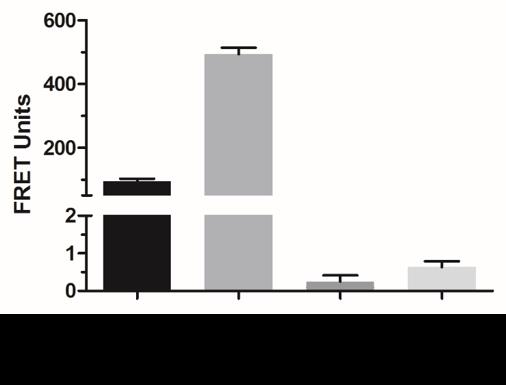
Figure 4. Binding of IRF5-CPPs to IRF5. THP-1 cells were incubated with FITC-IRF5-CPP2, FITC-IRF5-CPP5, FITC-IRF5-CPP8, or FITC-IRF5-CPP9 for 1 h, followed by permeabilization and staining for intracellular IRF5 with anti-IRF5 and tetramethyl rhodamine isothiocyanate (TRITC) antibodies. FRET units were calculated from fluorescence emissions.
Acknowledgments
Funding: This work was supported and funded by F. Hoffmann–La Roche, the Lupus Research Alliance (to B.J.B.), Department of Defense CDMRP Lupus Research Program W81XWH-18-1-0674 (to B.J.B.), NIH AR065959 (to B.J.B.), and EMD Serono Research and Development Institute Inc.
Competing interests
Disclosures: J.A.D., S.-L.T., and D.S. are inventors on patent application US20160009772A1 assigned to F. Hoffmann–La Roche AG. Application status abandoned as of 12 May 2019 as a matter of public record. Financial disclosures related to companies: G.C., C.-C.S., J.Q., M.D., and J.A.D. are employees of EMD Serono Research and Development Institute Inc. S.H. is employee of BMS. F.M. is the author of patent “Cell penetrating peptides & methods of identifying cell penetrating peptides” (WO2014001229A2) filed by F. Hoffmann–La Roche. J.A.D., N.F., A.F.H., K.-S.H., F.M., D.S., and S.-L.T. are authors of patent “Cell penetrating peptides which bind IRF5” (US20160009772A1) filed by Hoffmann–La Roche Inc.
References
- Banga, J., Srinivasan, D., Sun, C. C., Thompson, C. D., Milletti, F., Huang, K. S., Hamilton, S., Song, S., Hoffman, A. F., Qin, Y. G., Matta, B., LaPan, M., Guo, Q., Lu, G., Li, D., Qian, H., Bolin, D. R., Liang, L., Wartchow, C., Qiu, J., Downing, M., Narula, S., Fotouhi, N., DeMartino, J. A., Tan, S. L., Chen, G. and Barnes, B. J. (2020). Inhibition of IRF5 cellular activity with cell-penetrating peptides that target homodimerization.Sci Adv 6(20): eaay1057.
- De, S., Zhang, B., Shih, T., Singh, S., Winkler, A., Donnelly, R. and Barnes, B. J. (2017). B Cell-Intrinsic Role for IRF5 in TLR9/BCR-Induced Human B Cell Activation, Proliferation, and Plasmablast Differentiation. Front Immunol 8: 1938.
- Doucey, M. A., Goffin, L., Naeher, D., Michielin, O., Baumgartner, P., Guillaume, P., Palmer, E. and Luescher, I. F. (2003). CD3 delta establishes a functional link between the T cell receptor and CD8. J Biol Chem 278(5): 3257-3264.
- Song, S., De, S., Nelson, V., Chopra, S., LaPan, M., Kampta, K., Sun, S., He, M., Thompson, C. D., Li, D., Shih, T., Tan, N., Al-Abed, Y., Capitle, E., Aranow, C., Mackay, M., Clapp, W. L. and Barnes, B. J. (2020). Inhibition of IRF5 hyperactivation protects from lupus onset and severity. J Clin Invest 130(12): 6700-6717.
- Thompson, C. D., Matta, B. and Barnes, B. J. (2018). Therapeutic Targeting of IRFs: Pathway-Dependence or Structure-Based? Front Immunol 9: 2622.
- Ujlaky-Nagy, L., Nagy, P., Szollosi, J. and Vereb, G. (2018). Flow Cytometric FRET Analysis of Protein Interactions. Methods Mol Biol 1678: 393-419.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sherman, C. D. and Barnes, B. J. (2021). Intracellular IRF5 Dimerization Assay. Bio-protocol 11(10): e4021. DOI: 10.21769/BioProtoc.4021.
- Banga, J., Srinivasan, D., Sun, C. C., Thompson, C. D., Milletti, F., Huang, K. S., Hamilton, S., Song, S., Hoffman, A. F., Qin, Y. G., Matta, B., LaPan, M., Guo, Q., Lu, G., Li, D., Qian, H., Bolin, D. R., Liang, L., Wartchow, C., Qiu, J., Downing, M., Narula, S., Fotouhi, N., DeMartino, J. A., Tan, S. L., Chen, G. and Barnes, B. J. (2020). Inhibition of IRF5 cellular activity with cell-penetrating peptides that target homodimerization.Sci Adv 6(20): eaay1057.
Category
Biochemistry > Protein > Interaction > Protein-protein interaction
Biochemistry > Protein > Interaction > Protein-ligand interaction
Biochemistry > Protein > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










