- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Chromatographic Assays for the Enzymatic Degradation of Chitin
Published: Vol 11, Iss 9, May 5, 2021 DOI: 10.21769/BioProtoc.4014 Views: 5454
Reviewed by: Andrea PuharThomas HahnAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Simple Methods for the Preparation of Colloidal Chitin, Cell Free Supernatant and Estimation of Laminarinase
Ananthamurthy Koteshwara
Oct 5, 2021 5153 Views

Extraction and Electrophoretic Analysis of Bacterial Lipopolysaccharides and Outer Membrane Proteins
Yue-Jia Lee and Thomas J. Inzana
Dec 20, 2021 5752 Views

Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE
Zhe Wang [...] Chao Cai
Jun 20, 2025 3552 Views
Abstract
Chitin is an insoluble linear polymer of β(1→4)-linked N-acetylglucosamine. Enzymatic cleavage of chitin chains can be achieved using hydrolytic enzymes, called chitinases, and/or oxidative enzymes, called lytic polysaccharide monooxygenases (LPMOs). These two groups of enzymes have different modes of action and yield different product types that require different analytical methods for detection and quantitation. While soluble chromogenic substrates are readily available for chitinases, proper insight into the activity of these enzymes can only be obtained by measuring activity toward their polymeric, insoluble substrate, chitin. For LPMOs, only assays using insoluble chitin are possible and relevant. Working with insoluble substrates complicates enzyme assays from substrate preparation to product analysis. Here, we describe typical set-ups for chitin degradation reactions and the chromatographic methods used for product analysis.
Graphical abstract:
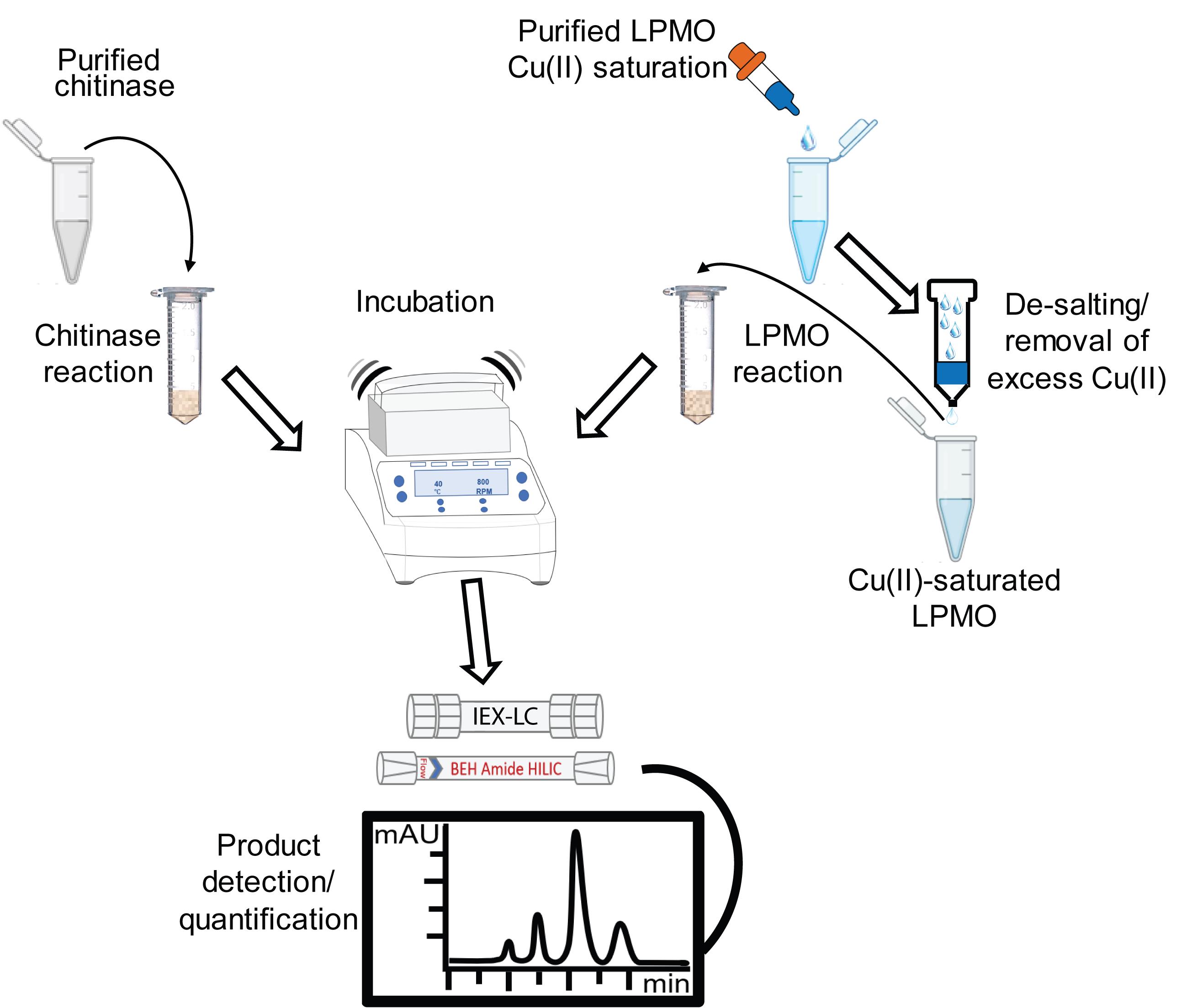
Overview of chromatographic methods for assessing the enzymatic degradation of chitin
Background
Chitin, an insoluble linear polymer of β(1→4)-linked N-acetylglucosamine, is one of the most abundant recalcitrant polysaccharides in nature, existing predominantly in two allomorphs. In α-chitin, the chains are organized in an anti-parallel fashion, which is the most common and recalcitrant form in nature; in β-chitin, the chains are organized in a parallel fashion, leading to a less recalcitrant structure with a higher water content (Gardner and Blackwell, 1975; Minke and Blackwell, 1978). Chitin is rarely found in its pure form in nature and is usually associated with minerals and proteins, and often with other polysaccharides as seen in fungal cell walls. Hence, obtaining pure chitin, e.g., from crustacean shells, requires demineralization and deproteination steps (Aye and Stevens, 2004). The efficiency of enzymatic saccharification of chitin depends on multiple factors: 1) the method used to extract chitin from the initial biomass; 2) the chitin particle size; and 3) the chitin form [e.g., Nakagawa et al. (2013)]. Thus, the choice of substrate for assaying enzymatic chitin degradation is far from trivial. It is noteworthy that the substrates used for assessing enzymatic chitin degradation, both here and in the field in general, are usually heavily processed and differ considerably from natural chitinous substrates.
Chitin may be converted to oligo- and/or mono-sugars by the coordinated action of multiple carbohydrate-active enzymes [CAZymes (Lombard et al., 2014)], which include several hydrolytic enzymes, such as chitinases (processive exo-chitinases and endo-chitinases; reviewed by Horn et al., 2006; Oyeleye et al., 2018), β-N-acetylhexosaminidases (reviewed by Slámová et al., 2010; also known as chitobiases], and oxidative metallo-enzymes called lytic polysaccharide monooxygenases [LPMOs (Vaaje-Kolstad et al., 2010)]. These enzymes differ in terms of their catalytic mechanism, substrate preferences (crystalline vs. amorphous, endo- vs. exo-attack), and product profiles (Vaaje-Kolstad et al., 2013). Chitinases cleave soluble chitooligosaccharides from chitin, the dominant product often being chitobiose. LPMOs (Chylenski et al., 2019) catalyze the oxidative cleavage of chitin chains, generating soluble and non-soluble C1-oxidized products. β-N-acetylhexosaminidases degrade soluble chitooligosaccharides produced by chitinases and LPMOs from the non-reducing end, producing N-acetylglucosamine and C1-oxidized chitobiose (chitobionic acid). In the CAZy database (Lombard et al., 2014), chitinases primarily occur in the glycoside hydrolase (GH) families GH18 and GH19. β-N-acetylhexosaminidases are found in GH family 20; however, enzymes possessing similar catalytic activity are also found in the GH families 3, 84, and 85. LPMOs are classified as Auxillary Activities (AAs), and chitin-active LPMOs have been detected in families AA10, AA11, and AA15 (Eijsink et al., 2019).
The active site of LPMOs consists of two highly conserved histidine residues that bind a single, catalytically crucial copper ion in a histidine brace (Quinlan et al., 2011; Chylenski et al., 2019). One of these histidines is the N-terminal residue (His1), which contributes to copper coordination with both its imidazole side chain and its (N-terminal) amino group. The latter implies that recombinantly produced LPMOs must be correctly processed, i.e., have an N-terminal histidine, to obtain an active enzyme (Eijsink et al., 2019). LPMOs require an oxygen-containing co-substrate (either O2 or H2O2) and a reductant for the reduction of copper (II) to copper (I). Chitin-active LPMOs appear to primarily target crystalline regions within chitin (Vaaje-Kolstad et al., 2010; Nakagawa et al., 2013), whereas chitinases are thought to prefer regions with a lower degree of substrate crystallinity. Characterization of LPMO activity faces multiple challenges related to the complex interplay among enzyme, reductant, oxidant, and substrate, as described in Eijsink et al., (2019). Importantly, LPMOs are prone to auto-catalytic inactivation (Bissaro et al., 2017; Loose et al., 2018), which obviously complicates the analysis of LPMO activity.
Proper characterization of the activity of chitinases or LPMOs, or combinations thereof, requires enzyme assays using a true polymeric substrate. While artificial chromogenic chitinase substrates are available and can be useful, measured activities toward such substrates provide only limited insight into the chitin-degrading ability of these enzymes. For chitin-active LPMOs, chromogenic artificial substrates do not exist and the very nature of these enzymes dictates that only assays using insoluble chitin are meaningful. Here, we describe methods for analyzing chitin degradation by bacterial LPMOs and chitinases. We also discuss points one should consider to successfully identify and evaluate the biochemical activities of these enzymes.
Materials and Reagents
2.0 ml microcentrifuge tubes (e.g., Axygen, catalog number: MCT-2000-C-S)
Single-channel mechanical pipettes, e.g.
0.2-2 µl (VWR, catalog number: 613-5258)
0.5-10 µl (VWR, catalog number: 613-5259)
2-10 µl (VWR, catalog number: 613-5260)
20-200 µl (VWR, catalog number: 613-5263)
100-1,000 µl (VWR, catalog number: 613-5265)
1,000-5,000 µl (VWR, catalog number: 613-5266)
Standard pipette tips, e.g.
Volume 0.1-10 µl (VWR, catalog number: 613-0735)
Volume 20-200 µl (VWR, catalog number: 613-0732)
Volume 100-1,250 µl (VWR, catalog number: 613-0739)
Volume 1,000-5,000 µl (VWR, catalog number: 613-0338)
Wide-orifice pipette tip refill system, e.g.
Volume 200 µl (VWR, catalog number: 732-3345)
Volume 1,000 µl (VWR, catalog number: 732-3348)
PD MidiTrap G-25 column (Cytiva LifeSciences, catalog number: 28918008)
Blue-capped flasks 25-1,000 ml (Fisher Scientific, DWK Life Sciences)
96-well filter plate (Millipore, catalog number: MSHVN4550) with MultiScreen Vacuum Manifold (Millipore, catalog number: MSVMHTS00)
Rezex RFQ-Fast Acid H+ (8%) 100 mm LC column (Phenomenex, catalog number: 00d-0223-k0)
Rezex RFQ-Fast Acid H+ (8%) 50 mm LC Guard column (Phenomenex, catalog number: 03b-0223-k0)
Rezex ROA-Organic Acid H+ (8%) 300 mm LC column (Phenomenex, catalog number: 00h-0138-k0)
0.3 ml polypropylene HPLC vials with caps and septa (Thermo Scientific, catalog number: 055428)
Bottle-top vacuum filtration PES filter, 0.2 or 0.45 µm (e.g., VWR, catalog number: 514-0338 or 514-0339)
Appropriate preparations of chitinases (e.g., Vaaje-Kolstad et al., 2013), chitobiase (e.g., Loose et al., 2014), and/or chitin-active LPMOs (e.g., Vaaje-Kolstad et al., 2010)
Tris (hydroxymethyl) aminomethane (Tris-HCl) (Sigma-Aldrich, catalog number: 1.08219)
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: SX0607N)
α-Chitin from shrimp shell (e.g., from Chitinor AS or from Sigma, catalog number: C9213)
β-Chitin from squid pen (Suppliers are France Chitin, and several Japanese and Chinese companies; the preparation process is described in Chaussard and Domard (2004))
BisTris (VWR, catalog number: 0715)
Ascorbic acid (Sigma-Aldrich, catalog number: A5960)
Copper (II) sulfate (Sigma-Aldrich, catalog number: 451657)
TraceSelect water (Fisher Scientific, catalog number: 95305)
N-acetylglucosamine (GlcNAc), purity ≥99% (Sigma-Aldrich, catalog number: A8625)
Diacetyl-chitobiose (GlcNAc2), purity >95% (Megazyme, catalog number: O-CHI2)
Triacetyl-chitotriose (GlcNAc3), purity >95% (Megazyme, catalog number: O-CHI3)
Tetraacetyl-chitotetraose (GlcNAc4), purity >95% (Megazyme, catalog number: O-CHI4)
Pentaacetyl-chitopentaose (GlcNAc5), purity >95% (Megazyme, catalog number: O-CHI5)
Hexaacetyl-chitohexaose (GlcNAc6), purity >95% (Megazyme, catalog number: O-CHI6)
Chitooligosaccharide oxidase from Fusarium graminearum as described by Heuts et al. (2008)
Sulfuric acid (Sigma-Aldrich, catalog number: 258105)
Hydrochloric acid, 37% (Merck, catalog number: 100317)
Acetonitrile (VWR, catalog number: 83640.400)
Ascorbic acid stock solution (see Recipes)
20 mM Tris pH 8.0 (see Recipes)
15 mM Tris pH 8.0 (see Recipes)
1 M BisTris pH 6.0 (see Recipes)
20 ml 50 mg/ml (w/v) chitin suspension (see Recipes)
50 mM H2SO4 (see Recipes)
5 mM H2SO4 (see Recipes)
Equipment
Magnetic stirrer (e.g., IKA RCT Basic, catalog number: 0003810000)
Stirring magnets, 25 mm (e.g., VWR, catalog number: 442-4524)
Security Guard cartridge and holder (Phenomenex, catalog numbers: aj0-4490 and kj0-4282)
Acquity UPLC BEH Amide column, 130 Å, 1.7 µm, 2.1 mm × 150 mm (Waters Corp., catalog number: 186004802)
Acquity UPLC BEH Amide VanGuard pre-column, 130 Å, 1.7 µm, 2.1 mm × 5 mm (Waters Corp., catalog number: 186004799)
pH meter (e.g., Metrohm, catalog number: 2.913.0210)
4°C refrigerator
-20°C freezer
Water purification system (MilliQ water)
Benchtop centrifuge (e.g., Eppendorf centrifuge 5418/5418R, catalog number: EP022620304)
Planetary ball-mill (e.g., Retsch, PM 100, catalog number: 20.540.0001)
Grinding jar (e.g., Retsch, Zirconium Oxide 500 ml, Comfort, catalog number: 01.462.0227)
Grinding balls (e.g., Retsch, Zirconium Oxide 10 mm Ø, catalog number: 22.455.0009)
Stainless-steel sieve, 0.8 mm (e.g.,Thermo Fisher Scientific, catalog number: 10739122)
Thermomixer C (Eppendorf, catalog number: 5382000015) with ThermoTop (Eppendorf, catalog number: 5308000003) and SmartBlock 1.5/2.0 ml (Eppendorf, catalog number: 5362000035)
Vacuum pump/compressor VCP 130 (VWR, catalog number: 181-0308)
UltiMateTM 3000 UHPLC system (Thermo Fisher Scientific) with the following central parts:
SRD-3200 Solvent Rack with 2 degasser channels (Thermo Fisher Scientific, catalog number: 5035.9250)
UltiMateTM ISO-3100BM Biocompatible Isocratic Pump (Thermo Fisher Scientific, catalog number: 5042.0011)
UltiMateTM TCC-3000RS Rapid Separation Thermostatted Column Compartment (Thermo Fisher Scientific, catalog number: 5730.0000)
WPS-3000 TSL Analytical Split-Loop Thermostatted Well Plate Autosampler (Thermo Fisher Scientific, catalog number: 5822.0020)
VWD-3100 Variable Wavelength Detector, one channel (Thermo Fisher Scientific, catalog number: 5074.0005)
UHPLC Agilent Technologies 1290 Infinity (Agilent Technologies Inc., catalog number: G4220-90301)
Software
Chromeleon data system, Chromeleon 7.2.9 (Thermo Fisher Scientific, https://www.thermofisher.com/order/catalog/product/CHROMELEON7#/CHROMELEON7)
Procedure
Unless otherwise stated, all solutions are prepared in standard purified water (MilliQ water). Enzymes are produced and purified as described elsewhere (e.g., Mekasha et al., 2020) and are usually stored at 4°C.
Preparation of copper-saturated LPMO
Note: All steps are performed at room temperature; see Notes below for challenges associated with using LPMOs in enzymatic reactions.
Mix a solution of Cu(II)SO4 in TraceSelect water (e.g., 50 mM) with a solution of purified LPMO (typically 50-100 μM, for example in 20 mM Tris pH 8.0) to obtain a 3-fold molar surplus of Cu(II) relative to the LPMO.
Incubate for 30 min at 4°C, without stirring.
To remove excess Cu(II), use a standard gravity flow size-exclusion chromatography protocol as described below (this protocol is slightly modified relative to supplier instructions, to minimize copper contamination in the final enzyme sample):
Equilibrate a PD MidiTrap G-25 desalting column by applying 15 ml 20 mM Tris pH 8.0 (enzyme storage buffer) in 5-ml aliquots. Allow the buffer to enter the packed column bed before adding the next portion.
Discard the flowthrough.
After the final 5-ml aliquot of washing buffer has entered the column, apply 0.5 ml Cu(II)-saturated LPMO solution; allow the solution to enter the packed bed completely.
Apply 0.5 ml 20 mM Tris pH 8.0 and allow the buffer to enter the packed bed completely.
Discard the flowthrough.
To elute the sample, place an microfuge tube under the column and apply 1.0 ml 20 mM Tris pH 8.0 to the column.
Collect the flowthrough, which contains the Cu(II)-saturated LPMO.
Discard the column.
Alternatively, excess free copper may be removed by standard high-performance size-exclusion chromatography using an appropriate chromatographic system. Dialysis is not recommended.
Preparation of substrate (see Notes below for challenges associated with using chitin as a substrate)
Mill the chitin to the desired particle size using a Retsch PM100 planetary ball-mill as described below:
Transfer chitin flakes to a 500-ml zirconium oxide grinding vessel containing zirconium oxide balls (10 × 10 mm) in (approximately) a 1:1 volume:volume ratio. Typically, with this vessel, one uses 100 ml chitin flakes and 100 ml milling balls; volumes are approximate and determined using a plastic graduated beaker. The appropriate total working volume for this vessel is 75-220 ml.
Mill the chitin using a milling speed of 450 rpm for a total milling period ranging from 15 to 30 min; to avoid excess heating, apply 5-min milling periods followed by 2-min pauses.
Pass the milled chitin through a Retsch 0.8-mm stainless-steel sieve and collect particles with a size ≤0.8 mm.
Transfer the milled chitin (particle size ≤0.8 mm) to a clean and dry container.
Store at room temperature.
Prepare a homogeneous stock suspension of the milled chitin (50 mg/ml) in TraceSelect water or a suitable buffer by mixing the suspension well for 24 h using magnetic stirring (see Recipes).
The suspension can be stored at 4°C. We recommend a maximum storage time of three months.
Activity assays
Chitinase assayStock solutions needed before starting the assays:
50 mg/ml chitin suspension prepared as described above.
1 M BisTris pH 6.0, where pH is set at 40°C.
Stock solution of purified chitinase, stored on ice. A typical concentration of such a stock solution would be 50 µM.
MilliQ water.
Prepare reactions (200 µl) as follows (volumes are based on the use of a 50 µM chitinase stock solution):
Using a wide-orifice pipette tip, transfer 40 µl magnetically stirred chitin suspension (final concentration of 10 mg/ml) to 2.0-ml reaction tubes.
Add 152 µl MiliQ water and 4 µl 1 M BisTris pH 6.0 (20 mM final concentration) to the chitin suspension.
Incubate the tubes in a pre-heated themomixer with ThermoTop at 40°C for 10 min.
Add 4 µl chitinase solution to the reaction tubes. This gives a final chitinase concentration of 1 µM, which tends to be appropriate; variations are possible and may be needed, depending on the enzyme type.
Incubate the reactions in a thermomixer with ThermoTop at 40°C, 1,000 rpm for the desired duration (typically 0.5-24 h; the optimal duration of the incubation depends on multiple factors, such as the efficiency of the chitinase(s), the type of chitin, and the purpose of the experiment).
The following stock solutions are needed in addition to those listed above:
100 mM ascorbic acid (see Recipes).
TraceSelect water (metal-free).
50 µM stock solution of purified and Cu(II)-saturated LPMO, prepared as described above and stored on ice.
Prepare reactions (200 µl) as follows:
Using a wide-orifice pipette tip, transfer 40 µl magnetically stirred chitin suspension (final concentration of 10 mg/ml) to 2.0 ml reaction tubes.
Add 150 µl TraceSelect water and 4 µl 1 M BisTris pH 6.0) (20 mM final concentration) to the chitin suspension.
Incubate the tubes in a pre-heated thermomixer with ThermoTop at 40°C for 10 min.
Add 4 µl Cu(II)-saturated LPMO (final concentration of 1 µM) to the reaction tubes.
Add 2 µl 100 mM ascorbic acid (1 mM final concentration).
Incubate the reactions in a thermomixer with ThermoTop at 40°C, 1,000 rpm for the desired duration (typically 0.5-24 h; the optimal duration of the incubation depends on multiple factors, such as the efficiency of the LPMO, the type of chitin, the amount and nature of the reductant, and the purpose of the experiment).
Sampling
Sampling of chitinase reactionsDraw 50 µl samples from the reaction tubes under shaking using wide-orifice pipette tips to ensure obtaining a homogenous suspension of chitin particles.
Immediately filter the samples using a 96-well filter plate and a multiScreen Vacuum Manifold connected to a vacuum pump/compressor. The filtrates are collected into another 96-well plate. This step should be well prepared and take less than 30 s.
Quench the reactions by mixing 20 µl filtrate (containing soluble products from the reaction) with 20 µl 50 mM H2SO4 (i.e., a two-fold dilution; see Recipes) in 0.3-ml polypropylene vials; seal with caps and septa.
Analyze the samples using the method described in Step E1 below.
Sampling of LPMO reactions and further processing for quantitation
To analyze the complete profile of soluble LPMO products, including longer oligosaccharides:
Follow steps 1 and 2 in “Sampling of chitinase reactions” above. This yields filtrates (from 50 μl reaction samples) containing soluble products.
Mix 13 µl filtrate with 37 µl 100% acetonitrile in a microfuge tube (i.e., a final dilution factor of approximately 4).
Spin the mixtures at max speed using an Eppendorf centrifuge 5418/54 to pellet the enzyme precipitate.
Transfer the supernatants to 0.3-ml polypropylene vials, seal with cap and septum.
Analyze the samples using the method described in Step E2 below.
To simplify quantitation of soluble LPMO products, further degradation of oxidized chitooligosaccharides by chitobiase (CHB) is recommended. The degradation by CHB yields the oxidized dimer, GlcNAc2ox, and the monomer, GlcNAc, as final products.
Follow steps 1 and 2 in “Sampling chitinase reactions” in Section D. This yields filtrates (from 50 µl reaction samples) containing soluble products.
Mix the filtrate (containing soluble LPMO products) with a solution of purified CHB, to reach a final CHB concentration of 2 µM. For example, mix 38 µl filtrate with 2 µl 40 µM CHB solution; see Loose et al. (2014) for details.
Incubate for 16 h at 37°C without shaking.
Spin the reaction tubes containing the CHB degradation products for a few seconds at max speed using an Eppendorf centrifuge 5418/54 to collect condensation droplets.
Transfer 20 µl reaction mixtures to 0.3-ml polypropylene vials containing 20 µl 50 mM H2SO4 (giving a total dilution factor of 2.1), mix well before sealing with caps containing septa.
Analyze the samples using the method described in Step E1 below.
Product analysis and quantitation
Analysis of native products from chitinase reactions and CHB-treated LPMO reactions (Figure 1).
If analyzing a mixture of native monomers and dimers, or a mixture of oxidized dimers and native monomers, HPLC analysis of the samples may be achieved using a Rezex RFQ-Fast Acid H+ (8%) column heated to 85°C, mounted in an UltimateTM 3000 UHPLC system, with 5 mM H2SO4 as the mobile phase (see Recipes) at a flow rate of 1 ml/min. The solutes are separated isocratically over 6 min and detected using UV absorption at 194 nm.
Note: The native trimer, tetramer, pentamer, and hexamer are also visible using this method, but the separation of these analytes is suboptimal (Figure 1).
If analysis of longer chitooligosaccharides is desired, a Rezex ROA-Organic H+ (8%) column heated to 65°C may be used, with the same system settings as described in point a above, except that the flow rate should be 0.6 ml/min. With this set-up, isocratic elution over 15 min gives better separation of the trimer, tetramer, and pentamer peaks as compared with the method described in a and shown in Figure 1. See Monge et al. (2018) for examples of chromatograms.
The amounts of GlcNAc1-6 and GlcNAc2ox are quantitated using GlcNAc1-6 and GlcNAc2ox standards of known concentrations, which need to be analyzed in the same round of chromatographic runs (same sample series). See Section F for preparation of the standards.
Analysis of chromatograms and calculations of analyte concentrations are performed using the Chromeleon 7.2.9 software.
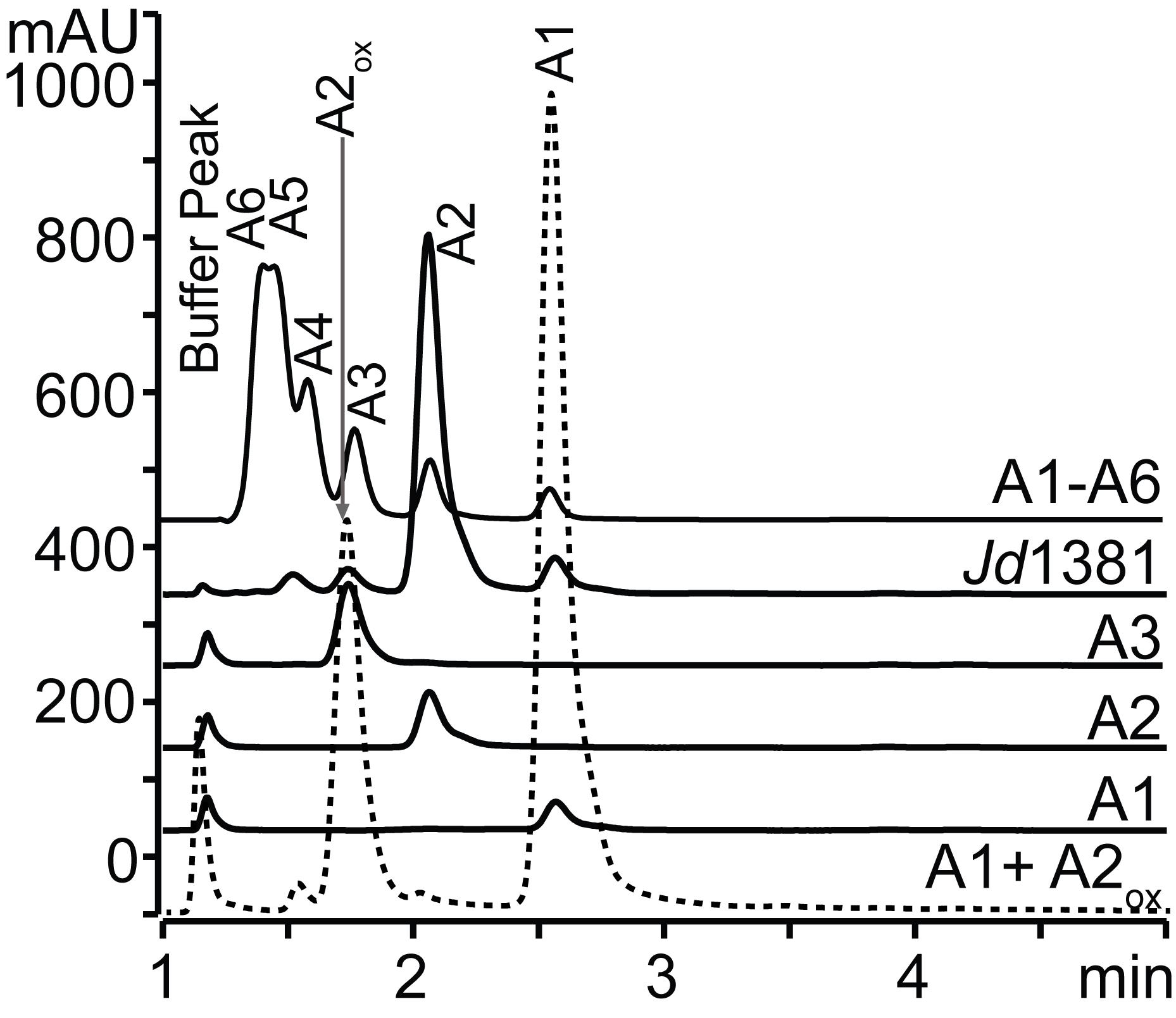
Figure 1. Examples of chromatograms produced using the method described in Step E1a. Peaks for native GlcNAc1-6, indicated as A1-A6, and the peak for oxidized chitobiose, indicated as A2ox, are labeled. The chromatograms show four standards (A1, A2, A3, and A1-A6), the products of a chitinase reaction (“Jd1381”), and the products of an LPMO reaction that has been treated with CHB (dotted line, showing the native monomer and the oxidized dimer). The figure is modified from Mekasha et al. (2020). The Jd1381 chitinase also contains an LPMO domain, and since the reaction was run with a reductant, the product mixture also contained small amounts of oxidized products, which elute prior to A2, whose poorly resolved peaks overlap with those belonging to longer non-oxidized products. mAU stands for milli-absorbance units.Analysis of product profiles from LPMO-containing reactions (Figure 2).
Note: This method can also be used to analyze the product profile of reactions with only chitinases if the separation of chitooligosaccharides using the (faster) methods described in Section E1 is considered insufficient. In this case, samples from the chitinase reactions need to be treated as described for the LPMO reactions (i.e., dilution in acetonitrile).
Analyze the samples by HPLC using an Acquity UPLC BEH Amide column mounted in an UHPLC Agilent Technologies 1290 Infinity system with a gradient composed of 15 mM Tris-HCl, pH 8.0, and 100% acetonitrile, as follows: 0-5 min, 74% acetonitrile; 5-7 min, 74-62% acetonitrile; 7-8 min, 62% acetonitrile; 8-10 min, 62-74% acetonitrile; 10-12 min, 74% acetonitrile.
Quantitate the amounts of oxidized products using a standard curve based on standards produced as described below. The chromatograms are analyzed using the Chromeleon 7.2.9 software.
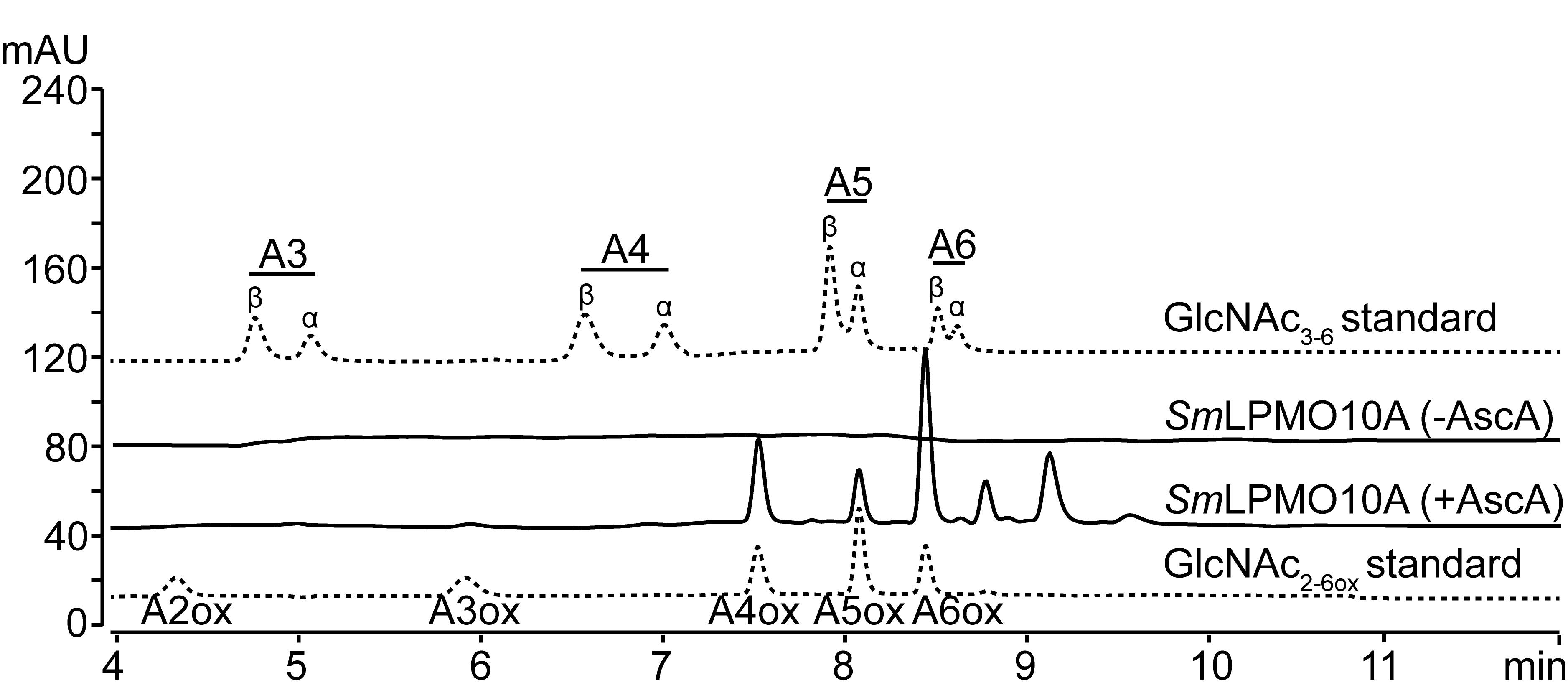
Figure 2. Examples of chromatograms produced using the method described in Step E2. Peaks for native GlcNAc3-6 are labeled A3-A6. Peaks representing oxidized products are labeled A2ox-A6ox. The dotted lines represent standards of native and oxidized chitooligosaccharides. Note that the α- and β-anomers of the native chitooligosaccharides are separated. The solid lines are examples of product profiles obtained after chitin degradation by a chitin-active LPMO (SmLPMO10A) in the presence or absence of a reductant, ascorbic acid (AscA). The figure is modified from Mekasha et al. (2020).
Production of chitooligosaccharide standards, including GlcNAc2ox, GlcNAc3ox, GlcNAc4ox, GlcNAc5ox, or GlcNAc6ox.
Preparation of oxidized chitooligosaccharides.
Mix 5 mM GlcNAc2, GlcNAc3, GlcNAc4, GlcNAc5 or GlcNAc6, or a mixture of these, with 2 µM purified chitooligosaccharide oxidase from Fusarium graminearum (Heuts et al., 2008; Loose et al., 2014).
Incubate at room temperature for 16 h without stirring; this leads to full conversion. The products in the reaction mixture are stable for months when frozen at -20°C.
Prepare standard solutions in 74% acetonitrile at concentrations in the range of 0.02 mM to 0.5 mM to generate standard curves for quantitation.
Spin the mixtures at max speed using an Eppendorf centrifuge 5418/54 to remove enzyme precipitate.
Transfer the supernatants to 0.3-ml polypropylene vials; seal with caps and septa.
Standard solutions for native products are prepared by diluting the chitooligosaccharide solutions made in point 1 to yield the appropriate concentrations. The required concentrations depend on the experiment and will usually be higher than those of the standards for oxidized products.
Notes
Chitin-related challenges
The lack of standard procedures for chitin isolation and pretreatment (e.g., milling) leads to variation among the chitins used in different laboratories, which affects the enzyme activity data obtained.
Since chitin is insoluble, it is important, and not straightforward, to produce homogenous chitin suspensions. Special care should be taken to have homogenous chitin suspensions both when setting up and when sampling reactions. The use of smaller particle sizes, e.g., below 1 mm in diameter, makes it easier to obtain homogenous suspensions and to pipette accurately. It is advisable to use wide-bore pipette tips (such as VWR’s wide-orifice pipette tips mentioned in the protocols above). When working with stock suspensions, magnetic stirring tends to be sufficient to keep the suspensions homogeneous.
Note that the particle size and crystallinity of the chitin affect the activity of chitin-degrading enzymes.
Enzyme-related challenges
While chitinases are “regular” hydrolytic enzymes that do not require special measures, working with LPMOs poses multiple challenges, as outlined in detail in Eijsink et al., (2019). A few key points are:
The LPMO reaction is dependent on a reductant, and different reductants may lead to very different reaction kinetics [e.g., Hegnar et al. (2019)]. Ascorbic acid is most frequently used.
LPMOs use H2O2, which may be added to the reaction or generated within the mixture by reactions involving O2 and the reductant (e.g., ascorbic acid). Special care should be taken to supply sufficient O2 (or H2O2) and reductant for the entire reaction time. This is not a problem when using the reaction conditions described above.
Special care should be taken to avoid inactivation of LPMOs, which may occur when using excess amounts of reductant and/or adding high amounts of H2O2.
LPMO activity and stability decrease if the enzyme is not fully copper-saturated.
The various redox processes in LPMO reactions, such as generation of H2O2 in the reaction mixture, will be affected by the presence of free transition metals in the solution, to an extent that depends on the reductant that is being used. Reactions with ascorbic acid are strongly affected by the presence of free copper ions (Bissaro et al., 2020).
Recipes
Ascorbic acid stock solution (100 mM)
Transfer 17.6 mg ascorbic acid to an Eppendorf tube
Keep the tube on ice and dissolve the ascorbic acid in 1 ml TraceSelect water by pipetting the mixture up and down
Prepare 20-µl aliquots in Eppendorf or PCR tubes and store at -20°C
Use the aliquots only once
500 ml 20 mM Tris pH 8.0
Weigh 1.211 g Tris (Mw 121.14 g/mol) and dissolve in approximately 400 ml MilliQ-water
Adjust the pH to 8.0 using HCl and then add MilliQ water to a total volume of 500 ml in a volumetric flask
Filter through a 0.45-µm or 0.22-µm bottle-top PES filter
Store at room temperature
500 ml 15 mM Tris pH 8.0
Weigh 0.909 g Tris (Mw 121.14 g/mol) and dissolve in approximately 400 ml MilliQ-water
Adjust the pH to 8.0 using HCl and then add MilliQ water to a total volume of 500 ml in a volumetric flask
Filter through a 0.45-µm or 0.22-µm bottle-top PES filter
Store at room temperature
500 ml 1 M BisTris pH 6.0
Weigh 104.62g BrisTris (Mw 209.24 g/mol) and dissolve in approximately 400 ml MilliQ-water
Adjust the pH to 6.0 using HCl and then add MilliQ water to a total volume of 500 ml in a volumetric flask
Filter through a 0.45-µm or 0.22-µm bottle-top PES filter
Store at room temperature
20 ml 50 mg/ml (w/v) chitin suspension
Weigh 1.0 g chitin and add 20 ml Trace Select water or a suitable buffer
Mix the suspension for 24 h by magnetic stirring at 4°C
Store at 4°C
50 ml 50 mM H2SO4
Add approximately 40 ml MilliQ-water to a 50-ml volumetric flask
Add 136 µl H2SO4 (Mw 98.08, 1.83 g/cm3)
Mix well and add MilliQ water to a total volume of 50 ml
Store at room temperature
2 L 5 mM H2SO4
Add approximately 1.9 L MilliQ-water to a 2-L volumetric flask
Add 544 µl H2SO4 (Mw 98.08, 1.83 g/cm3)
Mix well and add MilliQ water to a total volume of 2 L
Store at room temperature
Acknowledgments
We thank former and current lab members for their contributions to the development of our protocols. The present protocol is derived from Mekasha et al., J Biol Chem, 2020, 295:9134-9146, which describes results from projects 221576 and 247001, funded by the Research Council of Norway.
Competing interests
The authors declare no competing interests.
Ethics
There are no ethical issues associated with this protocol.
References
- Aye, K. N. and Stevens, W. F. (2004). Improved chitin production by pretreatment of shrimp shells. J Chem Technol Biot 79(4): 421-425.
- Bissaro, B., Røhr, A. K., Müller, G., Chylenski, P., Skaugen, M., Forsberg, Z. and Eijsink, V. G. H. (2017). Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nature Chem Biol 13(10): 1123-1128.
- Bissaro, B., Kommedal, E., Røhr, Å. K. and Eijsink, V. G. H. (2020) Controlled depolymerization of cellulose by light-driven lytic polysaccharide oxygenases. Nature Commun 11(1): 890.
- Chaussard, G. and Domard, A. (2004) New aspects of the extraction of chitin from squid pens. Biomacromolecules 5(2): 559-564.
- Chylenski, P., Bissaro, B., Sørlie, M., Røhr, Å. K., Várnai, A., Horn S. J. and Eijsink, V. G. H. (2019) Lytic polysaccharide monooxygenases in enzymatic processing of lignocellulosic biomass. ACS Catal 9(6): 4970-4991.
- Eijsink, V. G. H., Petrovic, D., Forsberg, Z., Mekasha, S., Røhr, Å. K., Várnai, A. and Vaaje-Kolstad, G. (2019). On the functional characterization of lytic polysaccharide monooxygenases (LPMOs). Biotechnol Biofuels 12: 58.
- Gardner, K. H. and Blackwell, J. (1975). Refinement of the structure of β-chitin. Biopolymers 14(8): 1581-1595.
- Hegnar, O. A., Petrovic, D. M., Bissaro, B., Alfredsen, G., Várnai, A. and Eijsink, V. G. H. (2019). pH-Dependent relationship between catalytic activity and hydrogen peroxide production shown via characterization of a Lytic Polysaccharide Monooxygenase from Gloeophyllum trabeum. Appl Environ Microbiol 85(5): e02612-18.
- Heuts, D. P., Winter, R. T., Damsma, G. E., Janssen, D. B. and Fraaije, M. W. (2008). The role of double covalent flavin binding in chito-oligosaccharide oxidase from Fusarium graminearum. Biochem J 413(1): 175-183.
- Horn, S. J., Sikorski, P., Cederkvist, J. B., Vaaje-Kolstad, G., Sørlie, M., Synstad, B. and Eijsink, V. G. H. (2006). Costs and benefits of processivity in enzymatic degradation of recalcitrant polysaccharides. Proc Natl Acad Sci USA 103(48): 18089-18094.
- Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. and Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42(Database issue): D490-495.
- Loose, J. S., Forsberg, Z., Fraaije, M. W., Eijsink, V. G. H. and Vaaje-Kolstad, G. (2014) A rapid quantitative activity assay shows that the Vibrio cholerae colonization factor GbpA is an active lytic polysaccharide monooxygenase. FEBS Lett 588(18): 3435-3440.
- Loose, J. S. M., Arntzen, M. O., Bissaro, B., Ludwig, R., Eijsink, V. G. H. and Vaaje-Kolstad, G. (2018). Multipoint precision binding of substrate protects lytic polysaccharide monooxygenases from self-destructive off-pathway processes. Biochemistry 57(28): 4114-4124.
- Mekasha, S., Tuveng, T. R., Askarian, F., Choudhary, S., Schmidt-Dannert, C., Niebisch, A. and Eijsink, V. G. H. (2020). A trimodular bacterial enzyme combining hydrolytic activity with oxidative glycosidic bond cleavage efficiently degrades chitin. J Biol Chem 295(27): 9134-9146.
- Minke, R. and Blackwell, J. (1978). The structure of alpha-chitin. J Mol Biol 120(2): 167-181.
- Monge, E. C., Tuveng, T. R., Vaaje-Kolstad, G., Eijsink, V. G. H. and Gardner, J. G. (2018). Systems analysis of the glycoside hydrolase family 18 enzymes from Cellvibrio japonicus characterizes essential chitin degradation functions. J Biol Chem 293(10): 3849-3859.
- Nakagawa, Y. S., Eijsink, V. G. H., Totani, K. and Vaaje-Kolstad, G. (2013). Conversion of α-chitin substrates with varying particle size and crystallinity reveals substrate preferences of the chitinases and lytic polysaccharide monooxygenase of Serratia marcescens. J Agr Food Chem 61(46): 11061-11066.
- Oyeleye, A. and Normi, Y. M. (2018). Chitinase: diversity, limitations, and trends in engineering for suitable applications. Biosci Rep 38(4): BSR2018032300.
- Quinlan, R. J., Sweeney, M. D., Lo Leggio, L., Otten, H., Poulsen, J. C., Johansen, K. S. and Walton, P. H. (2011). Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci USA 108(37): 15079-15084.
- Slámová, K., Bojarová, P., Petrásková, L. and Kren, V. (2010). β-N-acetylhexosaminidase: what's in a name? Biotechnol Adv 28(6): 682-693.
- Vaaje-Kolstad, G., Horn, S. J., Sørlie, M. and Eijsink, V. G. H. (2013). The chitinolytic machinery of Serratia marcescens-a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J 280(13): 3028-3049.
- Vaaje-Kolstad, G., Westereng, B., Horn, S. J., Liu, Z., Zhai, H., Sørlie, M. and Eijsink, V. G. H. (2010). An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330(6001): 219-222.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Mekasha, S., Tuveng, T. R., Vaaje-Kolstad, G. and Eijsink, V. G. H. (2021). Chromatographic Assays for the Enzymatic Degradation of Chitin. Bio-protocol 11(9): e4014. DOI: 10.21769/BioProtoc.4014.
- Mekasha, S., Tuveng, T. R., Askarian, F., Choudhary, S., Schmidt-Dannert, C., Niebisch, A. and Eijsink, V. G. H. (2020). A trimodular bacterial enzyme combining hydrolytic activity with oxidative glycosidic bond cleavage efficiently degrades chitin. J Biol Chem 295(27): 9134-9146.
Category
Biochemistry > Carbohydrate > Polysaccharide
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









